Male circumcision protects against urinary tract infections
John N. Krieger 2
1 School of Medical Sciences, University of Sydney, Sydney, Australia
2 Section of Urology, University of Washington, Seattle, United States
Abstract
Urinary tract infections (UTI) are common, debilitating and can have serious health consequences. UTI can present with severe local and systemic symptoms. Measures to reduce UTI occurrence deserve serious attention. We conducted a systematic review of the literature to evaluate data on the association between male circumcision (MC) and UTI. An extensive meta-analysis found that the lifetime risk of UTI in uncircumcised males is 32%, compared with 8.8% in circumcised males (relative risk reduction 3.7-fold). A number of studies suggest that phimosis may also be a risk factor for UTI. Thus, the number needed to treat (circumcise) is 4.3 and the single risk factor of lack of circumcision confers a 23% lifetime probability of UTI. Relative to circumcised males, UTI risk in uncircumcised males in the first year of life is 9.9, from age 1–16 years the relative risk is 6.6, and beyond age 16 years the relative risk is 3.4. The prevalence of UTI greatly exceeds the incidence of adverse events associated with the circumcision procedure (0.4% in infancy and 1.5% or more in older males). Most adverse events are minor. Because of the potential seriousness of UTI, high lifetime UTI incidence among uncircumcised males, and the strong protection afforded by circumcision, this simple, safe procedure (best performed early in infancy) should be more widely adopted. Circumcision confers an extensive array of other benefits, and has no adverse effect on sexual function or pleasure. Current evidence suggests that male circumcision should be regarded as a highly beneficial public health intervention similar to childhood vaccination.
Key points
- UTI is common, affecting 1 in 3 uncircumcised males over their lifetime
- Circumcision of males, preferably early in infancy, is highly protective against UTI
- The benefit in reduction of UTI by circumcision exceeds the procedural risk by 50:1
- UTI can lead to renal parenchymal disease
- Increases in antibiotic resistance adds to the challenges of treating pediatric and later age UTI
- Adopting ways of reducing UTI prevalence in males should be regarded as desirable
- Male circumcision has other lifetime benefits and is cost-saving for disease reduction
1 Introduction
The extensive medical and health benefits of male circumcision are now widely recognized and encapsulated in evidence-based policy statements [1], [2], [3], [4]. These include protection against several common sexually transmitted infections (STIs), genital cancers in both sexes, as well as physical problems and penile inflammation [5]. Penile inflammatory conditions will be reviewed in chapter 8 of section 18. In this chapter we discuss urinary tract infection (UTI), a common condition seen in both childhood and in later in life, focusing on the protection that male circumcision provides. We also discuss whether the risks seen in a minority of males are sufficient to justify calls for infant male circumcision to be promoted and supported by medical bodies and governments.
2 Urinary tract infections
UTI can occur at any age, but in males UTIs are most common in infancy [6], outnumbering those seen in infant girls by 5 to 1 [7]. The risk of UTI in boys is greatest during the first month of life [8]. UTI is the most common (4–10%) serious bacterial illness in febrile infants under 60 days of age [9], [10]. In infant boys presenting with intense pain and fever, lack of circumcision should alert the physician to consider UTI as the possible cause [11]. While most UTIs can be readily treated, significant morbidity can occur under some circumstances [12]. In very young boys a UTI can often be severe and in extreme cases can even lead to sepsis and death [13]. By age 7 years at least 2% of uncircumcised boys have had at least one UTI, although the true proportion has been suggested as being 5% [14]. A study by the American Academy of Pediatrics of UTIs in 1,895 infants aged 29 to 60 days in 20 US centers found bacteremia in 6.5% [10]. Among patients with bacteremia adverse events occurred in 8.1%, as compared with 2.3% in those without bacteremia. The likelihood of adverse events in infants with UTI and fever were: shock (0.4%), bacterial meningitis (0.3%), admission to an intensive care unit (2.0%), need for ventilatory support (0.6%), need for surgery (0.2%), death (0.1%), and other complications (0.6%). Over a 10-year follow-up, hypertension was seen in 12.8% of children with a history of UTI and surgically corrected vesico-ureteral reflux [15], [16].
Uropathogenic Escherichia coli account for 70–95% of UTIs [17], the ascending route being the most common avenue of acquisition, although seeding from systemic infection and health-care associated infections also occur [16]. In the bladder, experiments in mice found that bacteria colonize and invade superficial cells to form biofilms that appear on the bladder surface as pod-like bulges in which the bacteria are encased in a polysaccharide-rich matrix surrounded by a protective shell of uroplakin [18]. Such events may explain persistence of bladder infections in the face of robust host defences and represent a source of recurrent UTI [19]. How often these events occur in humans is unclear. Antibiotic prophylaxis has only modest efficacy in reducing UTI rates in children [20].
Of infants with UTI, 47% have a radiographically-identified urinary tract abnormality [21]. Vesicoureteric reflux is most common, with 1–2% affected, and comprises 30–40% of children with UTI [22]. Children with normal urinary tracts or non-dilating reflux do not benefit from antibiotic prophylaxis. In a study of boys aged 3–34 months with reflux, antibiotic prophylaxis did not decrease bacterial colonization of the foreskin, leading the authors to recommend circumcision [23]. Moreover, increasing bacterial resistance and low adherence to prescribed medication, represent major obstacles to successful antibiotic prophylaxis [24]. Maternal antibiotics during pregnancy also increase the risk of resistant pathogens causing neonatal UTI [25]. In children with prenatal hydronephrosis, febrile UTI was seen by 1–31 months of age in 19%, with a hazard ratio of 3.2 [26]. In such children continuous antibiotic prophylaxis reduced recurrent UTI risk, especially if there was ureteral dilation, high-grade reflux, or and uretero-vesicular junction obstruction [27]. Continuous antibiotic administration to children under the age of 2 years with high-grade, but not low-grade, prenatal hydronephrosis halved the incidence of UTI [28].
3 Circumcison to prevent UTIs
3.1 Retrieval of publications
A search of the PubMed database on 8 Feb 2016 for articles matching one or more of the keywords “circumcision”, “circumcised or “uncircumcised” plus one or more keywords yielded, for “UTI”, 60, 26 and 32 articles for each respective keyword combination; for “urinary tract infection” 305, 78, 87 articles, respectively; and for “bacteriuria” 19, 13, 13 articles, respectively. The abstracts of each were used to judge whether they were of sufficient quality to merit inclusion. Criteria for inclusion were that the article had to include either non-duplicated original data or meta-analysis of original data, publication in a peer-reviewed journal, and publication in English. A total of 22 studies reporting original data on circumcision and UTI were selected for inclusion (tables 1 and 2), together with 4 meta-analyses of studies of circumcision and UTI. We retrieved the full text of each article for detailed review.
| Study | Location | Design | Population age range | UTI definition | Circumcision classification |
| Wiswell 1987 [32] | USA Army hospitals | Cohort 1975–1984 | Birth–1 year | Not stated (92% of cultures suprapublic) | Birth records |
| Herzog 1989 [38] | Boston Children's Hospital, USA | Case-control 1985–1986 | Birth–1 year | ≥105 CFU/ml | Medical records; letter to parents |
| Kashani 1989 [39] | UCSD Medical Ctr, USA | Case-control 1980–1985 | 1 month–2 years | ≥105 CFU/ml (catheter or suprapubic aspir) | Medical records |
| Crain 1990 [41] | New York, USA | Case-control 1982–1987 | <8 weeks | ≥104 CFU/ml (bag/cath) >102 (suprapub) |
Medical records |
| Rushton 1992 [43] | Washington, DC, USA | Case-control 1987–1988 | 2 wk–6 mo | ≥105 CFU/ml (clean catch) >104 (catheterized) |
Medical records (prospectively for circumcised) |
| Spach 1992 [81] | Seattle, WA, USA | Case-control (STI clinic urine culture) |
Adult (median age cases 30 Controls 32) |
≥105 CFU/ml midstream plus ≥1 symptoms |
Examination |
| Wiswell 1993 [33] | USA Army hospitals | Cohort 1985–1990 | Birth–1 year | Not stated | Birth records |
| Craig 1996 [49] | Sydney, Australia | Case-control 1993–1994 | Birth–4 years | ≥105 (suprapub or cath); >108 (midstream urine) |
Parents or examination |
| Kim 1996 [72] | Seoul, Korea | Case-control | <15 years | ≥105 CFU/ml | Examination |
| Shaw 1998 [44] | Philadelphia PA, USA | Case-control 1995–1996 (1 year) |
≤1 year (84% African American) | ≥105 CFU/ml (sterile urethral catheterization) | Not stated |
| To 1998 [35] | Ontario, Canada | Cohort 1993 (fiscal yr) | Birth–3 years | ICD 9 codes 590, 595, 597, 599 | Canad Class Code 76.0 (in 1st month) |
| Herndon 1999 [45] | 3 sites, USA | Case-control 1993–1998 | “Boys” | Society for Fetal Urol data sheets |
Society for Fetal Urol data sheets |
| Schoen 2000 [34] | Kaiser Hospitals, CA, USA | Retrospective cohort 1996–1997 | Birth–1 year | ICD-9 coding or outpatient clin rec |
ICD-9 coding |
| Nayir 2001 [80] | Istanbul, Turkey | Randomized controlled trial | 3 mo–10 yr who had a UTI | ≥105 CFU/ml + symptom | Performed as part of trial |
| Newman 2002 [37] | 219 sites, USA | Case-control 1995–1998 | Birth–98 days | ≥102 CFU/ml (suprapubic); ≥2x104 (cath); ≥105 (bag, clean voided) |
Not stated |
| Kwak 2004 [47] | Seoul, Korea | Cohort 1985–1993 | 4.2–174 months | ≥105 CFU/ml | Performed during study |
| Zorc 2005 [9] | 8 sites, USA | Cross-sectional | ≤60 days | ≥103 CFU/ml (suprabubic); ≥5x104 (cath); ≥105 (cath plus +ve urinanalysis) |
Examination |
| Ghaemi 2007 [48] | Isfahan, Iran | Case-control Jul 2001–Feb 2002 | Neonates (mean 10.8 days) | Any CFU in suprapubic spec; or ≥104 in clean-voided |
Examination |
| Mukherjee 2009 [51] | Birmingham Children’s Hospital, UK | Retrospective cross-sectional case-note review | 1–18 years (mean 6.7) | Proven pure bacterial culture (organisms tabulated) | Not stated |
| Roth 2009 [40] | Children’s Hospital of Oklahoma, USA | Retrospective analysis | 1–11 months (mean 6.1 months) |
Positive urine culture | Not stated |
| Alsaywid 2010 [50] | Children’s Hospital Westmead, Sydney, Australia | Prospective cohort study 1995–2006 | 1 day–8.8 yr | Urine culture; organisms identified | Performed during study |
| Simforoosh 2012 [36] | Tehran, Iran | Prospective cohort study 2004–2008 | Neonatal followed for 15 months |
≥105 CFU/ml; if +ve or equivocal rechecked by suprapublic cath | Performed neonatally as part of study |
| Ref. | Circumcised | Uncircumcised | AORa, ARRb, ORc | Notes |
| Wiswell 1987 [32] | 151/173663 | 459/46112 | 11.4 (9.53–13.8) | e, h, i, j |
| Herzog 1989 [38] | 0/52 | 36/60 | 156 (9.22–26.60) | c, d, e, h, i, j |
| Kashani 1989 [39] | 1/43 | 16/83 | 10 (1.28–78.4) | c, e, f, h, i |
| Crain 1990 [41] | 4/96 | 18/103 | 4.87 (1.58–15) | c, e, h, i |
| Rushton 1992 [43] | 2/37 | 21/49 | 13.1 (2.83–60.8) | c, e, h, i |
| Spach 1992 [81] | 18/64 | 8/14 | 3.41 (1.04–11.2) | c, g, h, i |
| Wiswell 1993 [33] | 112/80279 | 384/27319 | 10.1 (8.17–12.4) | e, h, i, j |
| Craig 1996 [49] | 2/49 | 142/837 | 5.6 (1.4–20) | a, e, f, h, i |
| Kim 1996 [72] | 0/19 | 8/70 | 5.3 (0.293–96.1) | c, d, e, f, i |
| Shaw 1998 [44] | 6/497 | 6/75 | 7.12 (2.23–22.7) | c, e, i |
| To 1998 [35] | 55/29217 | 205/29217 | 3.7 (2.8–5) | b, e, f, h, i |
| Herndon 1999 [45] | 7/37 | 10/19 | 4.76 (1.41–16.1) | c, e, i |
| Schoen 2000 [34] | 22/9668 | 132/5225 | 11.1 (7.08–17.4) | e, h, i, j |
| Nayir 2001 [80] | 0/35 | 3/35 | 7 (0.375–131) | d, e, f, i |
| Newman 2002 [37] | 15/572 | 41/197 | 9.76 (5.26–18.1) | c, e, i |
| Kwak 2004 [47] | 6/27 | 18/50 | 1.97 (0.672–5.77) | c, f, i |
| Zorc 2005 [9] | 6/262 | 62/291 | 10.4 (4.7–31.4) | a, e, i |
| Ghaemi 2007 [48] | 2/105 | 16/148 | 6.24 (1.4–27.8) | c, e, i |
| Mukherjee 2009 [51] | –/NA | –/NA | 12 (6.4–23.6) | a, f, i |
| Roth 2009 [40] | 0/41 | 2/24 | 9.22 (0.424–201) | c, d, e, i |
| Alsaywid 2010 [50] | 5/74 | 62/137 | 11.4 (4.33–30) | c, e, f, i |
| Simforoosh 2012 [36] | 0/2000 | 20/1000 | 83.7 (5.05–1380) | c, d, e, f, h, i |
| The studies are listed in chronological order. In 'Notes' column: a = adjusted odds ratio; b = adjusted relative risk; c = odds ratio; d = small sample correction; e = infant; f = child; g = adult; h = general population; i = systematic search; j = USA. NA, not available. When a, b or c does not appear above it means that the study did not report one of these. |
||||
3.2 Infant UTI and circumcision
The first suggestion that male circumcision was associated with reduced UTI risk was in 1972 [29]. In 1982 Ginsburg and McCracken noticed that of 100 infants under 8 months of age with UTI, 95% were not circumcised [30]. Wiswell and co-workers examined the medical records for 1,919 circumcised and 583 uncircumcised boys born at Tripler Army Medical Center in Honolulu, Hawaii, from 1982–1983. They found the prevalence of UTI during infancy was 20 times higher among uncircumcised boys (0.21% vs. 4.12%, respectively) [31]. They then studied the birth records of 173,663 circumcised and 46,112 uncircumcised infant boys at US Army hospitals worldwide from 1975 to 1984. The key finding was that UTI rates were 11.4 fold higher in the uncircumcised group [32]. A further study of the medical records for 80,279 circumcised boys and 27,319 uncircumcised boys for the first year of life confirmed that UTI incidence rates during infancy were 10-fold higher among uncircumcised boys (1.4%) than among circumcised boys (0.14%) [33]. Another large US retrospective cohort study, at Kaiser Hospitals in California, included 9,668 circumcised and 5,225 uncircumcised infant boys monitored for 12 months. The key finding was that UTI incidence rates were 11.1-fold higher in the uncircumcised group (2.5%) than in the circumcised group (0.23%) [34].
A study in Ontario, Canada of 29,217 circumcised boys and 29,217 uncircumcised boys over the period from birth to age 3 years found that UTIs occurred 3.7 times more often among those who were uncircumcised [35]. A prospective cohort study of 2,000 boys circumcised in the newborn period and 1,000 uncircumcised boys followed for the first 15 months of life found that UTI incidence was 84-fold higher among uncircumcised infants [36].
The Pediatric Research in Office Settings’ Febrile Infant Study in the USA of 219 pediatric practices of infants younger than 3 months presenting with fever, diagnosed UTI in 2.6% of circumcised boys vs. 20.8% of uncircumcised boys [37]. In this study, being uncircumcised was the strongest UTI risk found in a multivariate analysis (OR=11.6; 95% CI=5.9–22.6).
The much higher UTI prevalence in uncircumcised male infants was confirmed in subsequent years by numerous smaller studies. These include studies from the Boston Children's Hospital [38], the University of California San Diego Medical Center [39], the Children's Hospital of Oklahoma [40], New York City [41], [42], Washington, DC [43], Philadelphia [44], multiple additional sites in the USA [45], [46], in Seoul, South Korea [47], Isfahan, Iran [48], Sydney, Australia [49], [50], and the Birmingham Children's Hospital, UK [51].
Israel appears to be an exception to the lower rate of UTI among male infants. In Israel, circumcision is performed by mohels (ritual circumcisers). UTI occurs commonly afterwards [52]. Hemostasis arising from tight bandaging of the penis post-circumcision to stem bleeding has been proposed as the cause, leading to recommendations that modifications to the traditional technique should be implemented [52], [53].
As indicated above, UTIs are especially common in uncircumcised boys with underlying urinary tract abnormalities [51], [54]. However, the risk reduction conferred by circumcision is 90% for both boys with normal urinary tracts and those with upper urinary tract abnormalities [55]. In patients with vesico-ureteral reflux antibiotic prophylaxis was not effective in reducing the bacterial colonization of the prepuce [56]. The authors recommended circumcision, particularly for boys with reflux, to reduce UTI risk. Prenatal hydronephrosis in infant males is associated with 3.6-fold higher UTI rate in uncircumcised compared with circumcised boys. Antibiotic prophylaxis did not reduce the UTI rate in male infants in this population [57]. Uncircumcised formula-fed infants with reflux were at higher UTI risk [58]. Early postnatal circumcision reduces UTI frequency in males with prenatal hydronephrisis [59]. In boys under 16 years of age with prenatal hydronephrosis and reflux, the incidence of UTI was 52% [60]. After circumcision the UTI rate decreased to 22%, compared to a UTI rate of 68% among boys who remained uncircumcised.
Amongst boys diagnosed with UTIs, recurrent UTI occurred in 19% of those who were not circumcised, but in none of those who were circumcised [61]. Recurrent UTI was seen in 39% of boys aged <2 years and 61% of boys aged 2 to 6 years (peaking at 3–5 years), although the period from first UTI to recurrence was not reported.
Some studies suggest that phimosis may further increase UTI risk [7], [62], [63], [64], [65], supporting the view that UTI pathogenesis involves bacteria ascending from the preputial sac where uropathogens accumulate [66], [67], [68]. Retractability of the foreskin is low among newborns, but common in adolescence [69]. A Japanese study found that presence of a nonretractile foreskin increased UTI risk 7.8-fold [62]. In contrast, a Canadian study of 440 boys, found similar UTI prevalence in uncircumcised boys with a completely visible (30%) vs. a partially or nonvisible (24%) urethral meatus [70].
Of infants diagnosed with their first UTI and anatomically normal urinary systems, recurrent UTI occurred in 34% of uncircumcised boys with nonretractile foreskins compared with 18% among boys whose foreskins could be retracted [7]. Presence of acute pyelonephritis was another risk factor for recurrent UTI in this study. Amongst infants born prematurely, a US study found that those who were uncircumcised had an 11-fold higher risk of UTI (95% CI 3.3–29; P<0.001) and that risk of UTI recurrence was eliminated by circumcision [71].
3.3 Circumcision and UTI in older boys
After infancy the prevalence of UTI in boys is lower. To and co-workers studied Canadian boys up to 3 years of age [35]. The inclusion of older boys, by diluting the UTI rate in infancy, would contribute to the rate in uncircumcised boys being 3.7 times higher than the rate in circumcised boys. A study in Sydney, Australia of 144 boys under 5 years of age (mean 0.5 years) found microbiologically proven UTIs in 47 (6.3%) of those who were uncircumcised vs. 2 (1.4%) of those who were circumcised (P=0.02) [49]. Other studies in older boys included another in Sydney of boys from infancy to age 8.8 years [50], two in Seoul, South Korea of boys under 15 years [72], [47], one in the USA of boys whose ages were not specified [45], and a UK study of boys aged 1 to 18 years [51]. The cumulative incidence of UTI was 2.2% by age 2 in a Swedish study [73], 1.8% by age 6 in Sweden [74], 6% in uncircumcised vs. 1.4% (n=2) in circumcised boys by age 5 years in a study in Western Sydney [49], and 3.6% to age 16 in a UK study [75]. The latter data were for specialist referrals and may be underestimates, since many UTIs are treated in primary care settings, which could include pediatricians, nurse practitioners and others in addition to general practitioners.
Bacterial colonization was found under the foreskin of over 98% of uncircumcised boys aged 2.9–7.4 years, more than 86% of the bacteria being uropathogenic [76]. After circumcision, bacterial colonization rates dropped from 100% to 86.3% (83.6% uropathogenic) in boys under 5 years of age and from 98.6% to 77.1% (70% uropathogenic) in boys aged 5 years or more. Foreskin swabs taken before circumcision from boys circumcised at an average age of 5.7 years (range 2 months to 9 years) contained 72 microorganisms, including 54 gram-positive bacteria (57% Enterococcus spp.) and 17 gram-negative bacteria (41% E. coli) and Candida spp. [76]. Bacteria cultured from periurethral swabs taken from healthy males who were uncircumcised (mean age 26.5 years) and circumcised (mean age 8.3 years) comprised gram negative rods in 17% vs. 4%, respectively, gram positive cocci in 62% vs. 80%, while streptococci, strict anaerobes and genital mycoplasmas were only present in the uncircumcised [77]. In boys aged from newborn to 13 years (mean 5.8 years), three yeast species were present under the foreskin of 12%. After circumcision this rate decreased to 1% of boys [78]. Swabs taken under the foreskin of boys aged 7 days to 11 years identified 50 bacterial isolates, most of which were multi-drug-resistant strains [79]. Taken together, these data suggest that uncircumcised males are more likely to harbour uropathogenic and antibiotic-resistant bacteria in their subpreputial space that are likely to be important in the pathogenesis of UTI.
A small randomized controlled trial was conducted in Istanbul, Turkey, of 88 boys aged 3 months to 10 years [80]. This population included 18 whose parents did not want them to be circumcised and 70 boys whose parents requested circumcision. All were followed for 6 months with monthly urine cultures. When a positive urine culture was detected antibiotics were administered. Of the 70 boys slated for circumcision, 35 receiving immediate circumcision and 35 had delayed circumcision 6 months later. In the delayed circumcision group bacteriuria was maintained until their circumcision. The rate plunged 25-fold after circumcision, the level becoming similar to that seen in the boys circumcised immediately after UTI diagnosis. A drawback of this study was the very small number of 6 symptomatic UTI cases. Clearly, much larger studies would be helpful.
3.4 Circumcision and UTI in men
In 80 young (median age 30 years) men attending STI clinics in Seattle, Washington, 26 had microbiologically confirmed UTI and 52 (median age 32 years) had urinary symptoms but negative urine cultures [81]. Eight (31%) of the 26 bacteriuric men presented as uncircumcised compared with 6 (12%) of the 52 nonbacteriuric men (odds ratio 3.4; 95% CI 1.0, 11.2; P=0.037). Among 19 men with gram-negative bacilli, 8 (42%) were uncircumcised vs. 6 (12%) of 52 nonbacteruric men (odds ratio 5.6; 95% CI 1.6, 19.4; P=0.004 [81]. Based on presentation of only 38 previously healthy university men with symptomatic UTIs, prevalence was considered low in another Seattle study [82]. Of these, 33 were circumcised and 5 were uncircumcised, indicating a similar rate of UTI in each. The number of cases were, however, too low to infer whether circumcision had a protective effect.
In elderly men UTI is common [83]. While genitourinary tract instrumentation (catheter placement and urological procedures) is a well-recognized risk factor for UTI [84], it is plausible that lack of circumcision might also contribute to UTI risk in elderly men. It is surprising that we could find no reports comparing UTI rates among elderly circumcised and uncircumcised men. Since nursing homes constitute a setting in which environmental factors are reasonably standardized for all occupants, studies of men in care facilities should be considered.
4 Meta-analyses of effect of circumcision on UTI rate
Meta-analyses have consistently noted strong protection conferred by infant circumcision against UTI [33], [85], [86], [87]. In 1992 the first meta-analysis conducted included 6 studies involving a total of 221,799 male infants [86]. The key finding was that being uncircumcised conferred a 13.1-fold higher UTI risk (95% CI 10.9–15.7) [86]. In 1993, Wiswell and Hachey [33] published a meta-analysis of male circumcision and UTIs in 209,399 infants from 9 studies. They found a 12.0-fold increase in UTI risk among uncircumcised boys (95% CI 10.6–13.6; range, 5–89-fold) [33]. A subsequent meta-analysis by Singh-Grewal and co-workers of 12 studies totalling 402,908 boys, including one randomized controlled trial, 4 cohort studies and 7 case-control studies found that circumcised boys had 87% lower UTI risk than uncircumcised boys (odds ratio 0.13, 95% CI 0.08–0.20), with the odds ratio being similar for each type of study design [85]. That meta-analysis was criticized by Schoen, who suggested that, “studies of older children and men [were] inappropriately included in the analysis,” and, “may explain why the odds ratio in boys is not as favourable as in earlier studies” [13]. Singh-Grewal et al. [85] recommended that circumcision should only be performed on boys with recurrent UTI or vesico-ureteric reflux. This recommendation was denounced by Schoen as being, “analogous to postponing immunization of an infant until the child is exposed to the pathogen or is diagnosed with the disease” [13]. Schoen regarded the newborn period as a, “window of opportunity” for circumcision because of simplicity, speed, faster healing, use of local anesthetic, and 10-times lower cost [13]. He also pointed out that, “the 2% complication rate mentioned is high”, noting that, “the American Academy of Pediatrics stated that complications of newborn circumcision are 'rare and usually minor' and that complications occur at a rate of 0.2% to 0.6% – [which is] 3 to 10 times lower than the rate cited by Singh-Grewal et al.” [13]. In addition, Schoen criticized the authors’ failure to point out that circumcision also protects against phimosis and balanitis during infancy and childhood, facilitates cleanliness, in young men helps prevent certain sexually transmitted infections (including human immunodeficiency virus and human papillomavirus) and protects against penile cancer later in life as well as cervical cancer in female sexual partners [13].
The most recent meta-analysis (in 2013) – that included data from 22 studies (Figure 1) involving a total of 407,903 males (296,837 circumcised and 111,066 uncircumcised) – determined the level of protection at three different ages of life – age 0–1 year, age 1–16 years and age >16 years (table 3) [87]. In infancy the relative risk of UTI was 9.9-fold higher in uncircumcised than in circumcised males (95% CI 7.5–13.1). For ages 1–16 years the relative risk of UTI was 6.6-fold higher in uncircumcised than in circumcised males (95% CI 3.3–13.2). For age >16 years the relative risk of UTI was 3.4-fold higher in uncircumcised than in circumcised males (95% CI 0.92–12.7) [87]. The study calculated that 32.1% (95% CI 15.6–49.8) of uncircumcised males experience a UTI over their lifetime compared with 8.8% (95% CI 4.2–13.2) of circumcised males (relative risk 3.7; 95% CI 1.1–11.8). The number needed to treat (circumcise) to prevent one case of UTI was 4.3 (95% CI 2.2–27.2). By subtracting 8.8 from 32.1 it was calculated that the single risk factor of lack of circumcision conferred a 23.3% risk of UTI over the lifetime [87]. No relevant original studies have been published since that meta-analysis so there is currently no need for an updated meta-analysis.
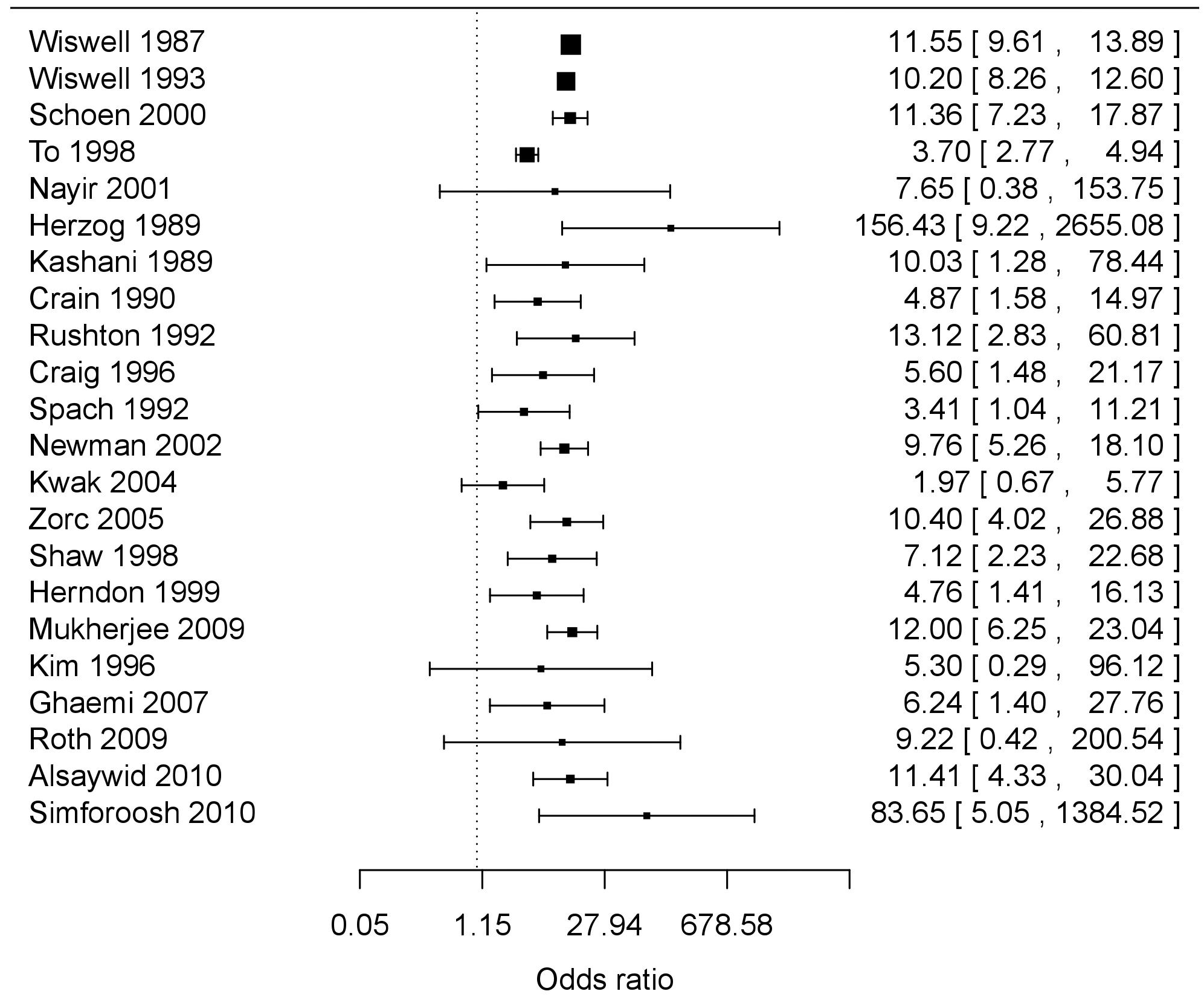
Mean is depicted as a square symbol and the first number immediately to the right of the symbol in each case.
Horizontal bars and numbers in brackets show the 95% confidence intervals.
| Age group | RR (95% CI) | Circ risk (95% CI) | Uncirc risk (95% CI) |
| 0–1 years | 9.91 (7.49–13.1) | 0.127% (0.072%–0.223%) | 1.26% (0.737–2.14) |
| 1–16 years | 6.56 (3.26–13.2) | 0.409% (0.221%–0.704%) | 2.68% (1.67–4.13) |
| 16+ years | 3.41 (0.916–12.7) | 8.26% (3.61%–12.7%) | 28.2% (11.6–45.7) |
| Lifetime | 3.65 (1.15–11.8) | 8.8% (4.15%–13.2%) | 32.1% (15.6–49.8) |
| Note that this Table does not include results for both meta-regression and stratified meta-analysis models, nor an analysis of various subsets such as studies of a general population versus those with VUR. | |||
5 UTI and risk of kidney damage
The data outlined above show that circumcision of male neonates and infants provides substantial protection against UTI. The developing kidney is especially susceptible to renal injury and scarring [43], [88], and the potential long-term consequences of these [89]. Renal imaging showed 50–86% of children with febrile UTI and presumed pyelonephritis have renal parenchymal defects [90]. These persisted. UTI in the first year of life was accompanied by pyelonephritis in 34–70% of cases in one study [9] and 90% in another [91]. Following treatment of febrile infants for UTI, nuclear scans revealed scarring in 10–30% [92].
Estimates in 1996 suggest that infant circumcision prevented 20,000 cases of acute pyelonephritis in the USA annually [93]. Today that figure would be higher. Acute pyelonephritis is a major cause of renal scarring [94], with a likelihood of 36–52% following UTI [91], [95], [96], [97]. A meta-analysis of 1,280 patients aged 0–18 years who underwent renal-bladder ultrasonography found renal scarring in 15.5% assessed 5 months after a first UTI [98].
Although grade IV or V reflux has been found to be the strongest predictor of renal scarring (22-fold higher likelihood than in patients without reflux), renal scarring was present in only 4.1% of patients [98], consistent with earlier observations [99]. For most patients, high fever (≥39°C) and an organism other than E. coli were associated with high risk of renal scarring [98]. Recurrent UTI increases the risk of renal damage. In male infants with normal urinary systems, the incidence of recurrent UTI in those less than 6 months of age was 26%, decreasing to 7.7% for males aged 6–12 months [7]. Acute pyelonephritis is one of the biggest risk factors for recurrent UTI, increasing the prevalence 4.6-fold [7]. Parenchymal infection and inflammation, rather than reflux, are the prerequisites for renal scarring [96], [97], [99].
A 27-year follow-up study of pyelonephritis in childhood noted hypertension associated with hyperreninemia (high plasma renin activity) and hypernatremia in 10–20% of cases, consistent with renal involvement [100]. Hypertension was seen in 12.8% of children with a history of UTI and surgically corrected reflux, with 17% of the hypertension occurring in those with gross renal scarring [15]. However, the overall risk of hypertension has been found to be low two decades after a childhood UTI with or without renal scarring [101]. Renal scarring subsequent to a UTI can sometimes progress to renal insufficiency and end-stage renal disease [100], [102]. Taken together, not only is the prevalence of UTI highest in infancy, but it is a much more severe and generalized disease at this age [102] – with fever the predominant sign due to pyelonephritis. UTI in early infancy has also been associated with a 1.5 times higher risk of asthma [103]. These observations suggest that measures that prevent pediatric UTI merit consideration.
A meta-analysis found an alarming rise in ciprofloxacin-resistant E. coli in UTI, leading to caution on the use of this antibiotic for treatment [104]. The increasing prevalence of multi-resistant gram-negative bacteria is occurring in all age groups, including children, as highlighted in a recent systematic review and meta-analysis of resistance to commonly used antibiotics used to treat UTI in children globally [105]. For ampicillin, resistance was 53.4% in OECD countries and 79.8% in non-OECD countries, and for co-amoxiclav was 8.2% and 60.3%, respectively. Antibiotic resistance was 13 times more prevalent in children who had previously been prescribed antibiotics in primary care [105]. In Australia, multi-resistant isolates of E. coli rose from 4.5% in 2008 to 7.2% in 2010, requiring treatment of uncomplicated UTIs with intravenous instead of oral antibiotics [106]. The impact of multi-drug resistant bacteria on health care costs and sepsis will be substantial. Methicillin-resistant Stapylococus aureus in children is increasing in the community by 10% per year in the USA, being higher in infants aged less than 90 days (44 per 100,000) compared with older infants (11 per 100,000) and children (1–3 per 100,000) [107].
6 Risk of adverse events from circumcision and lack of circumcision in the newborn period
The benefits of circumcision must be weighed up against the risks. In large series in 1989 it was found that adverse events occurred after 0.19% of 100,157 circumcisions performed in the first month of life [108]. Most adverse events were minor and easily treatable, leading to complete resolution. In contrast, adverse events were seen in 0.24% of 35,929 uncircumcised infants. All adverse events in uncircumcised infants were related to UTIs and included 32 in infants with concomitant bacteremia, 3 with meningitis, 2 with renal failure and 2 who died [108].
Subsequent studies reported similar findings. The most recent and authoritative data came from a study in 2014 by the Centers for Disease Control and Prevention. This study documented adverse events associated with circumcision of 1.4 million males in the USA (93.3% who were circumcised during the newborn period) [109]. It found prevalence of adverse events in infancy was 0.14%. Virtually all of these were minor, easily treatable and led to complete resolution. Adverse events were 20-fold higher for circumcision of boys aged 1–9 years and 10-fold higher for males older than age 10 [109].
7 Conclusions
Based on the strong evidence, male circumcision substantially reduces the risk of UTI. An analysis by researchers at Johns Hopkins University found enormous cost savings from circumcision when considering just the protection against UTIs and STIs [110]. Protection in infancy against UTIs was emphasized in the 2012 American Academy of Pediatrics infant male circumcision policy recommendations [1], the draft recommendations by the US Centers for Disease Control and Prevention [2], the American Urological Association policy on circumcision [3] and the infant male circumcision policy of the Circumcision Academy of Australia [4]. Coupled with its other lifetime benefits [1], [3], [5], circumcision of all infant males would seem desirable from a public health perspective. As pointed out by the US Centers for Disease Control and Prevention [3], benefits of male circumcision exceed risks by 100 to 1 [5]. In the case of just UTI described herein, circumcision benefits exceed risk of an adverse event by 50:1. Early infant circumcision provides protection against adverse medical conditions comparable to many childhood vaccines [34]. For example the level of protection deemed acceptable for vaccines against influenza [111], [112], [113] justifies claims that circumcision in infancy might be regarded as a “surgical vaccine” [114], [115], [116]. The benefit of male circumcision is even greater when one considers that over their lifetime, approximately half of uncircumcised boys will suffer a medical condition caused by the retention of the foreskin [5].
References
[1] American Academy of Pediatrics Task Force on Circumcision. Male circumcision. Pediatrics. 2012 Sep;130(3):e756-85. DOI: 10.1542/peds.2012-1990[2] Centers for Disease Control and Prevention. Recommendations for Providers Counseling Male Patients and Parents Regarding Male Circumcision and the Prevention of HIV Infection, STIs, and Other Health Outcomes - Docket No. CDC-2014-0012. 2014 [cited Jan 5, 2015]. Available from: https://www.cdc.gov/hiv/pdf/policies/mcpubliccommentnotice.pdf
[3] American Urological Association. Circumcision. 2012 [cited Mar 10, 2015]. Available from: http://www.auanet.org/about/policy-statements/circumcision.cfm
[4] Morris BJ, Wodak AD, Mindel A, Schrieber L, Duggan KA, Dilly A, Willcourt RJ, Cooper DA, Lumbers ER, Russell CT, Leeder SR. Infant male circumcision: An evidence-based policy statement. Open J Prevent Med. 2012;2:79-82. DOI: 10.4236/ojpm.2012.21012
[5] Morris BJ, Bailis SA, Wiswell TE. Circumcision rates in the United States: rising or falling? What effect might the new affirmative pediatric policy statement have? Mayo Clin Proc. 2014 May;89(5):677-86. DOI: 10.1016/j.mayocp.2014.01.001
[6] Koyle MA, Barqawi A, Wild J, Passamaneck M, Furness PD 3rd. Pediatric urinary tract infections: the role of fluoroquinolones. Pediatr Infect Dis J. 2003 Dec;22(12):1133-7. DOI: 10.1097/01.inf.0000101849.11912.8e
[7] Shim YH, Lee JW, Lee SJ. The risk factors of recurrent urinary tract infection in infants with normal urinary systems. Pediatr Nephrol. 2009 Feb;24(2):309-12. DOI: 10.1007/s00467-008-1001-0
[8] Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008 Apr;27(4):302-8. DOI: 10.1097/INF.0b013e31815e4122
[9] Zorc JJ, Kiddoo DA, Shaw KN. Diagnosis and management of pediatric urinary tract infections. Clin Microbiol Rev. 2005 Apr;18(2):417-22. DOI: 10.1128/CMR.18.2.417-422.2005
[10] Schnadower D, Kuppermann N, Macias CG, Freedman SB, Baskin MN, Ishimine P, Scribner C, Okada P, Beach H, Bulloch B, Agrawal D, Saunders M, Sutherland DM, Blackstone MM, Sarnaik A, McManemy J, Brent A, Bennett J, Plymale JM, Solari P, Mann DJ, Dayan PS; American Academy of Pediatrics Pediatric Emergency Medicine Collaborative Research Committee. Febrile infants with urinary tract infections at very low risk for adverse events and bacteremia. Pediatrics. 2010 Dec;126(6):1074-83. DOI: 10.1542/peds.2010-0479
[11] Shaikh N, Morone NE, Lopez J, Chianese J, Sangvai S, D'Amico F, Hoberman A, Wald ER. Does this child have a urinary tract infection? JAMA. 2007 Dec;298(24):2895-904. DOI: 10.1001/jama.298.24.2895
[12] Chon CH, Lai FC, Shortliffe LM. Pediatric urinary tract infections. Pediatr Clin North Am. 2001 Dec;48(6):1441-59. DOI: 10.1016/S0031-3955(05)70385-0
[13] Schoen EJ. Circumcision for preventing urinary tract infections in boys: North American view. Arch Dis Child. 2005 Aug;90(8):772-3. DOI: 10.1136/adc.2004.066761
[14] Sureshkumar P, Jones M, Cumming RG, Craig JC. Risk factors for urinary tract infection in children: a population-based study of 2856 children. J Paediatr Child Health. 2009 Mar;45(3):87-97. DOI: 10.1111/j.1440-1754.2008.01435.x
[15] Wallace DM, Rothwell DL, Williams DI. The long-term follow-up of surgically treated vesicoureteric reflux. Br J Urol. 1978 Dec;50(7):479-84. DOI: 10.1111/j.1464-410X.1978.tb06195.x
[16] Ma JF, Shortliffe LM. Urinary tract infection in children: etiology and epidemiology. Urol Clin North Am. 2004 Aug;31(3):517-26, ix-x. DOI: 10.1016/j.ucl.2004.04.016
[17] Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997 Sep;11(3):551-81. DOI: 10.1016/S0891-5520(05)70373-1
[18] Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003 Jul;301(5629):105-7. DOI: 10.1126/science.1084550
[19] Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci USA. 2006 Sep;103(38):14170-5. DOI: 10.1073/pnas.0602136103
[20] Craig JC, Simpson JM, Williams GJ, Lowe A, Reynolds GJ, McTaggart SJ, Hodson EM, Carapetis JR, Cranswick NE, Smith G, Irwig LM, Caldwell PH, Hamilton S, Roy LP; Prevention of Recurrent Urinary Tract Infection in Children with Vesicoureteric Reflux and Normal Renal Tracts (PRIVENT) Investigators. Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med. 2009 Oct;361(18):1748-59. DOI: 10.1056/NEJMoa0902295
[21] Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age: a 10-year evaluation. Pediatr Infect Dis J. 2014 Apr;33(4):342-4. DOI: 10.1097/INF.0000000000000110
[22] Puri P, Gosemann JH, Darlow J, Barton DE. Genetics of vesicoureteral reflux. Nat Rev Urol. 2011 Aug;8(10):539-52. DOI: 10.1038/nrurol.2011.113
[23] Gücük A, Burgu B, Gökçe İ, Mermerkaya M, Soygür T. Do antibiotic prophylaxis and/or circumcision change periurethral uropathogen colonization and urinary tract infection rates in boys with VUR? J Pediatr Urol. 2013 Dec;9(6 Pt B):1131-6. DOI: 10.1016/j.jpurol.2013.04.014
[24] Brandström P, Hansson S. Long-term, low-dose prophylaxis against urinary tract infections in young children. Pediatr Nephrol. 2015 Mar;30(3):425-32. DOI: 10.1007/s00467-014-2854-z
[25] Arshad M, Seed PC. Urinary tract infections in the infant. Clin Perinatol. 2015 Mar;42(1):17-28, vii. DOI: 10.1016/j.clp.2014.10.003
[26] Braga LH, Farrokhyar F, D'Cruz J, Pemberton J, Lorenzo AJ. Risk factors for febrile urinary tract infection in children with prenatal hydronephrosis: a prospective study. J Urol. 2015 May;193(5 Suppl):1766-71. DOI: 10.1016/j.juro.2014.10.091
[27] Herz D, Merguerian P, McQuiston L. Continuous antibiotic prophylaxis reduces the risk of febrile UTI in children with asymptomatic antenatal hydronephrosis with either ureteral dilation, high-grade vesicoureteral reflux, or ureterovesical junction obstruction. J Pediatr Urol. 2014 Aug;10(4):650-4. DOI: 10.1016/j.jpurol.2014.06.009
[28] Braga LH, Mijovic H, Farrokhyar F, Pemberton J, DeMaria J, Lorenzo AJ. Antibiotic prophylaxis for urinary tract infections in antenatal hydronephrosis. Pediatrics. 2013 Jan;131(1):e251-61. DOI: 10.1542/peds.2012-1870
[29] Mann PG. Proteus urinary infections in childhood. J Clin Pathol. 1972 Jun;25(6):551. DOI: 10.1136/jcp.25.6.551-a
[30] Ginsburg CM, McCracken GH Jr. Urinary tract infections in young infants. Pediatrics. 1982 Apr;69(4):409-12.
[31] Wiswell TE, Smith FR, Bass JW. Decreased incidence of urinary tract infections in circumcised male infants. Pediatrics. 1985 May;75(5):901-3. DOI: 10.1016/S0022-5347(17)47483-0
[32] Wiswell TE, Enzenauer RW, Holton ME, Cornish JD, Hankins CT. Declining frequency of circumcision: implications for changes in the absolute incidence and male to female sex ratio of urinary tract infections in early infancy. Pediatrics. 1987 Mar;79(3):338-42.
[33] Wiswell TE, Hachey WE. Urinary tract infections and the uncircumcised state: an update. Clin Pediatr (Phila). 1993 Mar;32(3):130-4. DOI: 10.1177/000992289303200301
[34] Schoen EJ, Colby CJ, Ray GT. Newborn circumcision decreases incidence and costs of urinary tract infections during the first year of life. Pediatrics. 2000 Apr;105(4 Pt 1):789-93. DOI: 10.1542/peds.105.4.789
[35] To T, Agha M, Dick PT, Feldman W. Cohort study on circumcision of newborn boys and subsequent risk of urinary-tract infection. Lancet. 1998 Dec 5;352(9143):1813-6. DOI: 10.1016/S0140-6736(98)02392-7
[36] Simforoosh N, Tabibi A, Khalili SA, Soltani MH, Afjehi A, Aalami F, Bodoohi H. Neonatal circumcision reduces the incidence of asymptomatic urinary tract infection: a large prospective study with long-term follow up using Plastibell. J Pediatr Urol. 2012 Jun;8(3):320-3. DOI: 10.1016/j.jpurol.2010.10.008
[37] Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in office settings: the Pediatric Research in Office Settings' Febrile Infant Study. Arch Pediatr Adolesc Med. 2002 Jan;156(1):44-54. DOI: 10.1001/archpedi.156.1.44
[38] Herzog LW. Urinary tract infections and circumcision. A case-control study. Am J Dis Child. 1989 Mar;143(3):348-50. DOI: 10.1001/archpedi.1989.02150150106026
[39] Kashani IJ, Faraday MS. The risk of urinary tract infection in uncircumcised male infants. Int Pediatr. 1989; 4: 44-5.
[40] Roth CC, Hubanks JM, Bright BC, Heinlen JE, Donovan BO, Kropp BP, Frimberger D. Occurrence of urinary tract infection in children with significant upper urinary tract obstruction. Urology. 2009 Jan;73(1):74-8. DOI: 10.1016/j.urology.2008.05.021
[41] Crain EF, Gershel JC. Urinary tract infections in febrile infants younger than 8 weeks of age. Pediatrics. 1990 Sep;86(3):363-7.
[42] Kaluarachchi D, Kaldas V, Erickson E, Nunez R, Mendez M. When to perform urine cultures in respiratory syncytial virus-positive febrile older infants? Pediatr Emerg Care. 2014 Sep;30(9):598-601. DOI: 10.1097/PEC.0000000000000203
[43] Rushton HG, Majd M. Pyelonephritis in male infants: how important is the foreskin? J Urol. 1992 Aug;148(2 Pt 2):733-6; discussion 737-8. DOI: 10.1016/S0022-5347(17)36706-X
[44] Shaw KN, Gorelick M, McGowan KL, Yakscoe NM, Schwartz JS. Prevalence of urinary tract infection in febrile young children in the emergency department. Pediatrics. 1998 Aug;102(2):e16.
[45] Herndon CD, McKenna PH, Kolon TF, Gonzales ET, Baker LA, Docimo SG. A multicenter outcomes analysis of patients with neonatal reflux presenting with prenatal hydronephrosis. J Urol. 1999 Sep;162(3 Pt 2):1203-8. DOI: 10.1016/S0022-5347(01)68134-5
[46] Zorc JJ, Levine DA, Platt SL, Dayan PS, Macias CG, Krief W, Schor J, Bank D, Shaw KN, Kuppermann N; Multicenter RSV-SBI Study Group of the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Clinical and demographic factors associated with urinary tract infection in young febrile infants. Pediatrics. 2005 Sep;116(3):644-8. DOI: 10.1542/peds.2004-1825
[47] Kwak C, Oh SJ, Lee A, Choi H. Effect of circumcision on urinary tract infection after successful antireflux surgery. BJU Int. 2004 Sep;94(4):627-9. DOI: 10.1111/j.1464-410X.2004.05014.x
[48] Ghaemi S, Fesharaki RJ, Kelishadi R. Late onset jaundice and urinary tract infection in neonates. Indian J Pediatr. 2007 Feb;74(2):139-41. DOI: 10.1007/s12098-007-0006-1
[49] Craig JC, Knight JF, Sureshkumar P, Mantz E, Roy LP. Effect of circumcision on incidence of urinary tract infection in preschool boys. J Pediatr. 1996 Jan;128(1):23-7. DOI: 10.1016/S0022-3476(96)70423-7
[50] Alsaywid BS, Saleh H, Deshpande A, Howman-Giles R, Smith GH. High grade primary vesicoureteral reflux in boys: long-term results of a prospective cohort study. J Urol. 2010 Oct;184(4 Suppl):1598-603. DOI: 10.1016/j.juro.2010.04.021
[51] Mukherjee S, Joshi A, Carroll D, Chandran H, Parashar K, McCarthy L. What is the effect of circumcision on risk of urinary tract infection in boys with posterior urethral valves? J Pediatr Surg. 2009 Feb;44(2):417-21. DOI: 10.1016/j.jpedsurg.2008.10.102
[52] Toker O, Schwartz S, Segal G, Godovitch N, Schlesinger Y, Raveh D. A costly covenant: ritual circumcision and urinary tract infection. Isr Med Assoc J. 2010 May;12(5):262-5.
[53] Amir J. Ritual circumcision and urinary tract infection in Israel. Isr Med Assoc J. 2010 May;12(5):303-4.
[54] Jang HC, Lee KH, Park JS. Primary Vesico-Ureteral Reflux: Comparison of Factors between Infants and Children. Korean J Urol. 2011 Mar;52(3):206-9. DOI: 10.4111/kju.2011.52.3.206
[55] Bader M, McCarthy L. What is the efficacy of circumcision in boys with complex urinary tract abnormalities? Pediatr Nephrol. 2013 Dec;28(12):2267-72. DOI: 10.1007/s00467-013-2410-2
[56] Cascio S, Colhoun E, Puri P. Bacterial colonization of the prepuce in boys with vesicoureteral reflux who receive antibiotic prophylaxis. J Pediatr. 2001 Jul;139(1):160-2. DOI: 10.1067/mpd.2001.115017
[57] Zareba P, Lorenzo AJ, Braga LH. Risk factors for febrile urinary tract infection in infants with prenatal hydronephrosis: comprehensive single center analysis. J Urol. 2014 May;191(5 Suppl):1614-8. DOI: 10.1016/j.juro.2013.10.035
[58] Dacher JN, Mandell J, Lebowitz RL. Urinary tract infection in infants in spite of prenatal diagnosis of hydronephrosis. Pediatr Radiol. 1992;22(6):401-4; discussion 404-5. DOI: 10.1007/BF02013495
[59] Kose E, Yavascan O, Turan O, Kangin M, Bal A, Alparslan C, Sirin Kose S, Kuyum P, Aksu N. The effect of circumcision on the frequency of urinary tract infection, growth and nutrition status in infants with antenatal hydronephrosis. Ren Fail. 2013;35(10):1365-9. DOI: 10.3109/0886022X.2013.828263
[60] Evans K, Asimakadou M, Nwankwo O, Desai D, Cherian A, Mushtaq I, Cuckow P, Duffy P, Smeulders N. What is the risk of urinary tract infection in children with antenatally presenting dilating vesico-ureteric reflux? J Pediatr Urol. 2015 Apr;11(2):93.e1-6. DOI: 10.1016/j.jpurol.2015.01.009.
[61] Conway PH, Cnaan A, Zaoutis T, Henry BV, Grundmeier RW, Keren R. Recurrent urinary tract infections in children: risk factors and association with prophylactic antimicrobials. JAMA. 2007 Jul;298(2):179-86. DOI: 10.1001/jama.298.2.179
[62] Hiraoka M, Tsukahara H, Ohshima Y, Mayumi M. Meatus tightly covered by the prepuce is associated with urinary infection. Pediatr Int. 2002 Dec;44(6):658-62. DOI: 10.1046/j.1442-200X.2002.01633.x
[63] Lee JW, Cho SJ, Park EA, Lee SJ. Topical hydrocortisone and physiotherapy for nonretractile physiologic phimosis in infants. Pediatr Nephrol. 2006 Aug;21(8):1127-30. DOI: 10.1007/s00467-006-0104-8
[64] Tarhan H, Akarken I, Koca O, Ozgü I, Zorlu F. Effect of preputial type on bacterial colonization and wound healing in boys undergoing circumcision. Korean J Urol. 2012 Jun;53(6):431-4. DOI: 10.4111/kju.2012.53.6.431
[65] Matsuoka H, Kajiwara I, Tahara H, Oshima K. [Phimosis as a pathogenetic factor in urinary tract infection and vesicoureteral reflux]. Nippon Hinyokika Gakkai Zasshi. 1994 Jun;85(6):953-7. DOI: 10.5980/jpnjurol1989.85.953
[66] Günşar C, Kurutepe S, Alparslan O, Yilmaz O, Dağlar Z, Sencan A, Genç A, Taneli C, Mir E. The effect of circumcision status on periurethral and glanular bacterial flora. Urol Int. 2004;72(3):212-5. DOI: 10.1159/000077117
[67] Fussell EN, Kaack MB, Cherry R, Roberts JA. Adherence of bacteria to human foreskins. J Urol. 1988 Nov;140(5):997-1001. DOI: 10.1016/S0022-5347(17)41909-4
[68] Wiswell TE, Miller GM, Gelston HM Jr, Jones SK, Clemmings AF. Effect of circumcision status on periurethral bacterial flora during the first year of life. J Pediatr. 1988 Sep;113(3):442-6. DOI: 10.1016/S0022-3476(88)80625-5
[69] Ko MC, Liu CK, Lee WK, Jeng HS, Chiang HS, Li CY. Age-specific prevalence rates of phimosis and circumcision in Taiwanese boys. J Formos Med Assoc. 2007 Apr;106(4):302-7. DOI: 10.1016/S0929-6646(09)60256-4
[70] Dubrovsky AS, Foster BJ, Jednak R, Mok E, McGillivray D. Visibility of the urethral meatus and risk of urinary tract infections in uncircumcised boys. CMAJ. 2012 Oct;184(15):E796-803. DOI: 10.1503/cmaj.111372
[71] Cason DL, Carter BS, Bhatia J. Can circumcision prevent recurrent urinary tract infections in hospitalized infants? Clin Pediatr (Phila). 2000 Dec;39(12):699-703. DOI: 10.1177/000992280003901203
[72] Kim KK. Preputial condition and urinary tract infections. J Korean Med Sci. 1996 Aug;11(4):332-4. DOI: 10.3346/jkms.1996.11.4.332
[73] Jakobsson B, Esbjörner E, Hansson S. Minimum incidence and diagnostic rate of first urinary tract infection. Pediatrics. 1999 Aug;104(2 Pt 1):222-6.
[74] Mårild S, Jodal U. Incidence rate of first-time symptomatic urinary tract infection in children under 6 years of age. Acta Paediatr. 1998 May;87(5):549-52. DOI: 10.1111/j.1651-2227.1998.tb01502.x
[75] Coulthard MG, Lambert HJ, Keir MJ. Occurrence of renal scars in children after their first referral for urinary tract infection. BMJ. 1997 Oct;315(7113):918-9. DOI: 10.1136/bmj.315.7113.918
[76] Ladenhauf HN, Ardelean MA, Schimke C, Yankovic F, Schimpl G. Reduced bacterial colonisation of the glans penis after male circumcision in children--a prospective study. J Pediatr Urol. 2013 Dec;9(6 Pt B):1137-44. DOI: 10.1016/j.jpurol.2013.04.011
[77] Serour F, Samra Z, Kushel Z, Gorenstein A, Dan M. Comparative periurethral bacteriology of uncircumcised and circumcised males. Genitourin Med. 1997 Aug;73(4):288-90. DOI: 10.1136/sti.73.4.288
[78] Aridogan IA, Ilkit M, Izol V, Ates A, Demirhindi H. Glans penis and prepuce colonisation of yeast fungi in a paediatric population: pre- and postcircumcision results. Mycoses. 2009 Jan;52(1):49-52. DOI: 10.1111/j.1439-0507.2008.01535.x
[79] Anyanwu LJ, Kashibu E, Edwin CP, Mohammad AM. Microbiology of smegma in boys in Kano, Nigeria. J Surg Res. 2012 Mar;173(1):21-5. DOI: 10.1016/j.jss.2011.04.057
[80] Nayir A. Circumcision for the prevention of significant bacteriuria in boys. Pediatr Nephrol. 2001 Dec;16(12):1129-34. DOI: 10.1007/s004670100044
[81] Spach DH, Stapleton AE, Stamm WE. Lack of circumcision increases the risk of urinary tract infection in young men. JAMA. 1992 Feb;267(5):679-81. DOI: 10.1001/jama.1992.03480050083029
[82] Krieger JN, Ross SO, Simonsen JM. Urinary tract infections in healthy university men. J Urol. 1993 May;149(5):1046-8. DOI: 10.1016/S0022-5347(17)36292-4
[83] Shortliffe LM, McCue JD. Urinary tract infection at the age extremes: pediatrics and geriatrics. Am J Med. 2002 Jul 8;113 Suppl 1A:55S-66S. DOI: 10.1016/S0002-9343(02)01060-4
[84] Mitchell BG, Ferguson JK, Anderson M, Sear J, Barnett A. Length of stay and mortality associated with healthcare-associated urinary tract infections: a multi-state model. J Hosp Infect. 2016 May;93(1):92-9. DOI: 10.1016/j.jhin.2016.01.012
[85] Singh-Grewal D, Macdessi J, Craig J. Circumcision for the prevention of urinary tract infection in boys: a systematic review of randomised trials and observational studies. Arch Dis Child. 2005 Aug;90(8):853-8. DOI: 10.1136/adc.2004.049353
[86] Amato D, Garduño-Espinosa J. Circuncisión en el niño recién nacido y el riesgo de presentar infección de vías urinarias durante el primer año de vida. Un meta-análisis [Circumcision in the newborn child and risk of urinary tract infection during the first year of life. A meta-analysis]. Bol Med Hosp Infant Mex. 1992 Oct;49(10):652-8.
[87] Morris BJ, Wiswell TE. Circumcision and lifetime risk of urinary tract infection: a systematic review and meta-analysis. J Urol. 2013 Jun;189(6):2118-24. DOI: 10.1016/j.juro.2012.11.114
[88] Stull TL, LiPuma JJ. Epidemiology and natural history of urinary tract infections in children. Med Clin North Am. 1991 Mar;75(2):287-97. DOI: 10.1016/S0025-7125(16)30454-0
[89] Wiswell TE. The prepuce, urinary tract infections, and the consequences. Pediatrics. 2000 Apr;105(4 Pt 1):860-2.
[90] Rushton HG, Majd M. Dimercaptosuccinic acid renal scintigraphy for the evaluation of pyelonephritis and scarring: a review of experimental and clinical studies. J Urol. 1992 Nov;148(5 Pt 2):1726-32. DOI: 10.1016/S0022-5347(17)37014-3
[91] Rushton HG. Urinary tract infections in children. Epidemiology, evaluation, and management. Pediatr Clin North Am. 1997 Oct;44(5):1133-69. DOI: 10.1016/S0031-3955(05)70551-4
[92] Hoberman A, Wald ER, Hickey RW, Baskin M, Charron M, Majd M, Kearney DH, Reynolds EA, Ruley J, Janosky JE. Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics. 1999 Jul;104(1 Pt 1):79-86.
[93] Roberts JA. Neonatal circumcision: an end to the controversy? South Med J. 1996 Feb;89(2):167-71. DOI: 10.1097/00007611-199602000-00002
[94] Elder JS. Urinary tract infections. In: Kligeman RM, Behrman RE, Jenson HB, Stanton BF, editors. Textbook of Pediatrics. 18th edition. Philadelphia: Saunders Elsevier; 2007. p. 2223-8.
[95] Jakobsson B, Berg U, Svensson L. Renal scarring after acute pyelonephritis. Arch Dis Child. 1994 Feb;70(2):111-5. DOI: 10.1136/adc.71.4.386-b
[96] Benador D, Benador N, Slosman D, Mermillod B, Girardin E. Are younger children at highest risk of renal sequelae after pyelonephritis? Lancet. 1997 Jan 4;349(9044):17-9.
[97] Wallin L, Bajc M. Typical technetium dimercaptosuccinic acid distribution patterns in acute pyelonephritis. Acta Paediatr. 1993 Dec;82(12):1061-5.
[98] Shaikh N, Craig JC, Rovers MM, Da Dalt L, Gardikis S, Hoberman A, Montini G, Rodrigo C, Taskinen S, Tuerlinckx D, Shope T. Identification of children and adolescents at risk for renal scarring after a first urinary tract infection: a meta-analysis with individual patient data. JAMA Pediatr. 2014 Oct;168(10):893-900. DOI: 10.1001/jamapediatrics.2014.637
[99] Rushton HG. The evaluation of acute pyelonephritis and renal scarring with technetium 99m-dimercaptosuccinic acid renal scintigraphy: evolving concepts and future directions. Pediatr Nephrol. 1997 Feb;11(1):108-20.
[100] Jacobson SH, Eklöf O, Eriksson CG, Lins LE, Tidgren B, Winberg J. Development of hypertension and uraemia after pyelonephritis in childhood: 27 year follow up. BMJ. 1989 Sep;299(6701):703-6.
[101] Wennerström M, Hansson S, Jodal U, Stokland E. Primary and acquired renal scarring in boys and girls with urinary tract infection. The Journal of Pediatrics. 2000 Jan;136(1):30–4. DOI: 10.1016/S0022-3476(00)90045-3
[102] Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. The American Journal of Medicine. 2002 Jul;113(1):5–13. DOI: 10.1016/S0002-9343(02)01054-9
[103] Lin CH, Wang YC, Lin WC, Kao CH. Neonatal urinary tract infection may increase the risk of childhood asthma. Eur J Clin Microbiol Infect Dis. 2015 Sep;34(9):1773-8. DOI: 10.1007/s10096-015-2411-0
[104] Fasugba O, Gardner A, Mitchell BG, Mnatzaganian G. Ciprofloxacin resistance in community- and hospital-acquired Escherichia coli urinary tract infections: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2015 Nov;15:545. DOI: 10.1186/s12879-015-1282-4
[105] Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. BMJ. 2016 Mar;352:i939.
[106] Looke DF, Gottlieb T, Jones CA, Paterson DL. Gram-negative resistance: can we combat the coming of a new "Red Plague"? Med J Aust. 2013 Mar;198(5):243-4.
[107] Iwamoto M, Mu Y, Lynfield R, Bulens SN, Nadle J, Aragon D, Petit S, Ray SM, Harrison LH, Dumyati G, Townes JM, Schaffner W, Gorwitz RJ, Lessa FC. Trends in invasive methicillin-resistant Staphylococcus aureus infections. Pediatrics. 2013 Oct;132(4):e817-24. DOI: 10.1542/peds.2013-1112
[108] Wiswell TE, Geschke DW. Risks from circumcision during the first month of life compared with those for uncircumcised boys. Pediatrics. 1989 Jun;83(6):1011-5.
[109] El Bcheraoui C, Zhang X, Cooper CS, Rose CE, Kilmarx PH, Chen RT. Rates of adverse events associated with male circumcision in U.S. medical settings, 2001 to 2010. JAMA Pediatr. 2014 Jul;168(7):625-34. DOI: 10.1001/jamapediatrics.2013.5414
[110] Kacker S, Frick KD, Gaydos CA, Tobian AA. Costs and effectiveness of neonatal male circumcision. Arch Pediatr Adolesc Med. 2012 Oct;166(10):910-8. DOI: 10.1001/archpediatrics.2012.1440
[111] Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NJ; Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep. 2007 Jul 13;56(RR-6):1-54.
[112] Kelly H, Carville K, Grant K, Jacoby P, Tran T, Barr I. Estimation of Influenza Vaccine Effectiveness from Routine Surveillance Data. Galvani AP, editor. PLoS ONE. 2009 Mar 31;4(3):e5079. DOI: 10.1371/journal.pone.0005079
[113] Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. The Lancet Infectious Diseases. 2012 Jan;12(1):36–44. DOI: 10.1016/S1473-3099(11)70295-X
[114] Schoen EJ. Circumcision as a lifetime vaccination with many benefits. The Journal of Men’s Health & Gender. 2007 Sep;4(3):306–11. DOI: 10.1016/j.jmhg.2007.05.005
[115] Morris BJ. Why circumcision is a biomedical imperative for the 21(st) century. Bioessays. 2007 Nov;29(11):1147-58. DOI: 10.1002/bies.20654
[116] Ben KL, Xu JC, Lu L, Lü NQ, Cheng Y, Tao J, Liu DK, Min XD, Cao XM, Li PS. [Male circumcision is an effective "surgical vaccine" for HIV prevention and reproductive health]. Zhonghua Nan Ke Xue. 2009 May;15(5):395-402.




