Viral urological infections in kidney transplantation: BK polyomavirus (BKPyV)-associated diseases
Jennifer Kranz 2,3
1 Dept. of Urology, University Medical Center Rostock, Rostock, Germany
2 Dept. of Urology; St. Antonius Hospital Eschweiler; Germany
3 Dept. of Urology and Kidney Transplantation; Martin-Luther-University Halle (Saale); Germany
Abstract
Viral infections continue to be prevalent and a major problem in kidney transplant recipients. One of the most significant viruses for renal transplant patients is BK polyomavirus (BKPyV), the cause of BKPyV-associated nephropathy (BKVAN). BKVAN is seen in ca. 5% of renal transplant recipients and can lead to chronic allograft failure or even graft loss in up to 50% of cases. We aimed to summarize current evidence for screening and treatment of BKVAN and important findings for further research. Therefore, we summed up the evidence from well-established guidelines and searched ClinicalTrials.gov for ongoing randomized controlled trials (RCTs). Best evidence for screening for BKPyV is summarized by the American Society of Transplantation Infectious Diseases Community of Practice in 2019: They recommend screening for BKPyV viremia monthly, until month nine, and then every three months until two years post-transplant. Extended screening after two years may be considered in pediatric patients. Stepwise immunosuppression reduction is recommended for BKPyV viremia >1,000 copies/ml. There is no evidence for an effective reduction of immunosuppression or therapy for BKVAN available at the moment and high-quality studies or RCTs are missing, so further high-quality research is warranted. Promising new approaches for evaluation of therapy of BKVAN in RCTs are virus-specific T cells and targeting the viral immune response.
Summary of findings
- BK polyomavirus (BKPyV) is the most important polyomavirus for kidney transplant patients
- BKPyV-associated nephropathy (BKVAN) is the most critical disease in these patients: It is seen in 5% of renal transplant recipients and can lead to graft loss in up to 50% of cases
- Best evidence for screening for BKPyV is summarized by the American Society of Transplantation Infectious Diseases Community of Practice in 2019: They recommend screening for BKPyV viremia monthly, until month nine, and then every three months until two years post-transplant. Extended screening after two years may be considered in pediatric patients. Stepwise immunosuppression reduction is recommended for BKPyV viremia >1,000 copies/ml.
- There is no evidence for an effective reduction of immunosuppression or therapy for BKVAN available
- High-quality studies or RCTs are missing, so further high-quality research is warranted
- Promising new approaches for evaluation of therapy of BKVAN in RCTs are virus-specific T cells and targeting the viral immune response
1 Introduction
Viral infections continue to be prevalent and a major problem in kidney transplant recipients. Table 1 gives an overview of viral infections in renal transplantation according to the presenting time. These infections are especially virulent in patients with no previous contact with the virus (seronegative) or after increased immunosuppression, e.g. rejection treatment. Furthermore, recognition may be complicated by the tropism of some of the viruses for the allograft, thus deteriorating renal function and requiring differential diagnosis between viral infection and graft rejection. Complex antiviral strategies have been developed, mainly to deal with cytomegalovirus (CMV), including prophylaxis, pre-emptive therapy and therapy [1].
| Time to presentation | Early (1 month after transplantation) | >1 month to <6 months after transplantation |
Late (>6 months after transplantation) |
| Type of infection | Nosocomial related Donor acquired Latent |
Opportunistic | Community acquired Opportunistic |
| Virus | Herpes virus CMV |
Herpes virus CMV BKPyV EBV Influenza Parainfluenza virus RSV |
CMV Respiratory viruses |
| CMV = cytomegalovirus; BKPyV = BK polyomavirus; Epstein-Barr virus; RSV = Respiratory Syncytial Virus | |||
Polyomaviruses are of particular interest to kidney transplant recipients. They are small DNA viruses that were first discovered in 1971. At present, 13 different types are recognized but the most significant for renal transplant patients is BK polyomavirus (BKPyV), the cause of BKPyV-associated nephropathy (BKVAN). Furthermore, this virus can lead to hemorrhagic cystitis and ureter stenosis [1], [2]. To put it in a nutshell, BKPyV is the most important polyomavirus affecting renal transplant recipients, and adequate management of this infection may significantly impact on allograft survival. The general population is exposed to BKPyV during childhood, and 80–95% of the adults are seropositive. The virus persists in different cells from which it can be reactivated. Sometimes the infection may be transmitted with the allograft [1]. Although BKPyV has been detected in patients with heart or liver transplantation and patients with human immunodeficiency virus (HIV) infection or intestinal inflammatory disease, BKVAN is mainly described in renal transplant recipients [1], [3], [4], [5], [6].
BKVAN is seen in approximately 5% of renal transplant recipients and can lead to chronic allograft failure or even graft loss in up to 50% of cases. Some of the proposed risk factors include older age due to waning immunity, human leukocyte antigen (HLA) mismatches, acute rejection, steroid therapy and maintenance immunosuppression with tacrolimus [1], [7]. However, the common surrogate to all these factors is BKPyV viremia [1], [8].
The infection (also reactivation is possible) is initially asymptomatic, so surveillance programs are essential. The diagnosis then is based on quantitative polymerase-chain-reaction (PCR) in plasma (viremia) and urine (viruria) in the presence of acute renal failure. Unfortunately, no effective therapy is available [1].
Consequently, we aim to summarize the strategies to tackle BKVAN in kidney transplant patients from current guidelines, show results from recent randomized-controlled studies (RCT) for therapy and introduce ongoing trials in this book chapter.
2 Methods
Firstly, we searched the current guidelines on BKVAN screening and treatment, namely the European recommendations of the European Association of Urology (EAU), European Society of Clinical Microbiology and Infectious Diseases Study Group for Infection in Compromised Hosts (ESGICH), KDIGO (Kidney Disease Improving Global Outcome), Clinical practice guidelines for the care of kidney transplant recipients and the American Society of Transplantation Infectious Diseases Community of Practice.
Secondly, an evidence analysis was performed with a literature search in MEDLINE via PubMed for the period from January 2016 to 25th May 2021 [9]. We used the MeSH (Medical Subject Headings) Terms “BK virus” and “Kidney Transplantation”. Only RCTs and quasi-RCTs were included in the present analysis, case reports and reviews of all kinds were excluded. Additionally, we only included studies about screening or therapy of BKPyV-associated nephropathy following kidney transplantation (and no other transplantations), all other studies, e.g. with BKPyV-associated hemorrhagic cystitis, were excluded. Furthermore, we followed the recommendations provided in the PRISMA reporting guidelines [10].
Thirdly, we searched ClinicalTrials.gov for ongoing RCTs and quasi-RCTs about surveillance and treatment of BKPyV-associated nephropathy following kidney transplantation with the term “BK virus nephropathy” on 27th May 2021.
3 Results
3.1 Surveillance and treatment of BKPyV-associated nephropathy – recommendations in current guidelines in kidney transplantation
European recommendations suggest that viremia should be monitored systematically during the first six months after transplantation, but no effective therapy is available at the moment. Immunosuppression should be reduced with a switch to mammalian target of rapamycin (mTOR) inhibitors if possible. Leflunomide, intravenous immunoglobulins, foscavir, brincidofovir and bortezomib have shown promising results in at least some studies of BKVAN treatment [1], [11].
The European Society of Clinical Microbiology and Infectious Diseases Study Group for Infection in Compromised Hosts (ESGICH) focuses on their recommendations on special therapies to treat the BKPyV infections in the immunocompromised host [12]. To give an example, leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase and is approved to treat rheumatoid arthritis. Its immunosuppressive effects on T and B lymphocytes are through the selective inhibition of the mTOR signaling pathway. Moreover, an in-vitro inhibition of BKPyV viral DNA synthesis has been reported [13], [14], [15], [16], but ESGICH recommendation does not currently recommend leflunomide as a first-line treatment owing to the lack of definitive evidence (grade B, III) [12].
However, KDIGO Clinical practice guidelines for the care of kidney transplant recipients recommend an extended screening: Mostly, BKVAN occurs in the first two years after transplant with only 5% of cases occurring between two and five years after transplant. Accordingly, the timing and frequency of testing in recommended screening algorithms should reflect these data and balance the cost of screening with the potential to prevent BKVAN. Furthermore, it is stated that screening can reduce graft losses, because a reduction of immunosuppression can be done earlier. Still, the treatment recommendations for biopsy-proven BKVAN are unsatisfactory: reduction of immunosuppression does appear to have some impact on BKVAN, though variable rates of graft loss attributable to BKVAN have been reported even when reduction of immunosuppression has been employed. A common practice of immunosuppressive dose reduction is a withdrawal of antimetabolite (azathioprine or mycophenolate-mofetil) and reducing calcineurin inhibitors dosage by 50% [17], [18].
The newest guideline recommendations were published in 2019 by the American Society of Transplantation Infectious Diseases Community of Practice. They recommend screening for BKPyV viremia monthly, until month nine, and then every three months until two years post-transplant. Extended screening after two years may be considered in pediatric patients. Stepwise immunosuppression reduction is recommended for BKPyV viremia >1,000 copies/ml. Since properly randomized trials are lacking, there is no general recommendation for switching to certain immunosuppressive drugs [19].
3.2 Recent RCTs for surveillance and treatment of BKPyV-associated nephropathy in kidney transplantation
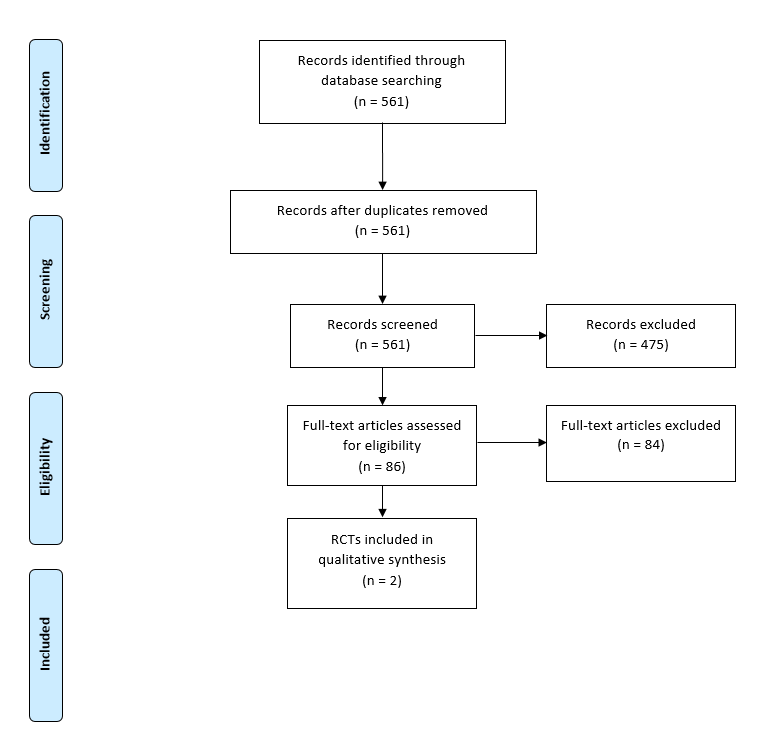
The literature search for primary studies yielded 561 results. Finally, two RCTs were included with a total of 240 patients (figure 1) [20], [21]. Table 2 shows the main characteristics, interventions, endpoints and results of the two studies included.
|
Reference |
Wojciechowski et al., 2017 [20] |
Patel et al., 2019 [21] |
|
Study Design |
Prospective single-center, randomized, open label pilot trial |
Prospective, randomized, double-blind, placebo-controlled trial |
|
Evidence |
SIGN: 1- |
SIGN: 1+ |
|
Participants |
40 patients, randomized in 1:1 manner; 11 patients in everolimus and 8 patients in MMF group reached primary endpoint |
200 kidney transplant recipients 2:1 (133 treatment group; 67 placebo group) |
|
Intervention |
MMF withdrawal with conversion to everolimus versus 50% reduction of MMF dose for treatment of BKPyV after kidney transplantation |
250 mg ciprofloxacin twice daily oral vs. placebo for 3 months |
|
Endpoints |
Primary endpoint was a >50% reduction of BKPyV viruria or clearance of viremia at 3 months post-randomization |
Primary endpoint safety and efficacy of ciprofloxacin for the prevention of BKPyV viremia in kidney transplant recipients; first 6 months post-transplant |
|
Main Results |
11 patients reached primary endpoint in everolimus and 8 in MMF group (p = 0.53). Of those with BKPyV viremia at time of enrolment, 8 of 16 and 5 of 15 cleared the viremia by month 3 in the everolimus conversation and MMF group (p = 0.47) |
Higher rates of BKPyV viremia (23.3% vs. 11.9%; p = 0.06) and BKPyV nephropathy (5.8% vs. 1.5%; p = 0.26) remained at 12 months in the ciprofloxacin group. Ciprofloxacin use was associated with significantly higher rate of fluoroquinolone-resistant gram-negative infections (83.3% vs. 50%; p = 0.04). |
|
Authors Conclusions |
Conversion from MMF to everolimus in BKPyV infection demonstrated a trend toward viral clearance, but did not reach statistical significance. |
A 3-month course of ciprofloxacin was ineffective at preventing BKPyV viremia in kidney transplant recipients and was associated with an increased risk of fluoroquinolone-resistant infections. |
| SIGN = Scottish Intercollegiate Guidelines Network; MMF mycophenolate mofetil; BKPyV = BK Polyomavirus |
||
Wojciechowski et al. performed an RCT about a switch in immunosuppression from mycophenolate mofetil (MMF) to everolimus in BKPyV, but this failed to show significant viral clearance [20]. On the whole, a switch from MMF to everolimus cannot be recommended to treat BKPyV-associated nephropathy, but the study is underpowered. Additionally, Patel et al. had also negative results for a 3-month course of ciprofloxacin to prevent BKPyV viremia [21].
Unfortunately, both current RCTs did not find an effective strategy to tackle BKPyV infection in kidney transplant recipients.
3.3 Ongoing RCTs for surveillance and treatment of BKPyV-associated nephropathy in kidney transplantation
ClinicalTrials.gov was searched for ongoing trials (RCT) about intervention BKPyV nephropathy, eleven registered studies have been identified. Two RCTs were still ongoing and recruiting. One trial from France named “Multicenter randomized two-arms study evaluating the BK viral clearance in kidney transplant recipients with BK viremia”. This study also used everolimus as a drug and has the NTC number 03216967. Another RCT from the United States uses a new drug called viralym and is named “Study of viralym-MLYR105 in kidney transplant recipients with BK viremia (NTC 04605484)”.
4 Further research
There is a lack of evidence for screening and adequate therapy of BKPyV infections, especially BKPyV-associated nephropathy, in kidney transplant patients. High-quality RCTs are missing and there are not many ongoing RCTs. The most knowledge on this topic arises from retrospective case series. Consequently, further studies are warranted, especially RCTs about the treatment and switch of immunosuppression. That is why it is difficult to give clear recommendations.
Luckily, we can learn from experimental retrospective and prospective studies and plan proper RCTs to solve the problem. There is evidence from experimental and clinical studies that virus-specific T cells could be used to monitor and treat BKPyV infection [7], [22], [23], [24]. Furthermore, other immunological targets, like pro-inflammatory cytokines could be promising approaches to treat BKPyV disease [25].
In summary, RCTs for screening and treating of BKVAN about virus-specific T cells and targeting the immune response are most promising and also highly needed.
5 Conclusions
BKPyV is the most important polyomavirus in renal transplant recipients and leads mostly to BKVAN. This disease has a high impact on allograft survival. Unfortunately, an optimal screening strategy for viruria and viremia is missing. Furthermore, there is no established therapy or even a therapy that can be highly recommended. Best evidence is summarized 2019 by the American Society of Transplantation Infectious Diseases Community of Practice. They recommend screening for BKPyV viremia monthly until month nine, and then every three months until two years post-transplant. Extended screening after two years may be considered in pediatric patients. Stepwise immunosuppression reduction is recommended for BKPyV viremia >1,000 copies/ml. In our opinion, this strategy should be performed.
Since new high-quality research is also sparse, research in this field is warranted and necessary. Promising new targets for further evaluation in research are virus-specific T cells and targeting the viral immune response.
6 Conflict of interest
Both authors declare that they have no conflict of interest regarding this manuscript.
References
[1] Figueiredo A, Lledó-García E, editors. European textbook on kidney transplantation. Arnhem: GLD Grafimedia; 2017.[2] Schneidewind L, Neumann T, Kranz J, Knoll F, Pelzer AE, Schmidt C, et al. Nationwide survey of BK polyomavirus associated hemorrhagic cystitis in adult allogeneic stem cell transplantation among haematologists and urologists. Ann Hematol. 2017 May;96(5):797-803. DOI: 10.1007/s00277-017-2935-8
[3] Loeches B, Valerio M, Perez M, Basnares R, Ledesma J, Fogeda M, et al. BK virus in liver transplant recipients: a prospective study. Transplant Proc. 2009 Apr;41(3):1033-7. DOI: 10.1016/j.transproceed.2009.02.021
[4] Loeches B, Valerio M, Palomo J, Bouza E, Muñoz P. BK virus in heart transplant recipients: a prospective study. J Heart Lung Transplant. 2011 Jan;30(1):109-11. DOI: 10.1016/j.healun.2010.08.028
[5] Flores V, Rodríguez-Sánchez B, Marín-Jiménez I, Bouza E, Menchén L, Muñoz P. Prospective study of BK virus infection in patients with inflammatory bowel disease. ScientificWorldJournal. 2014 Feb 13;2014:970528. DOI: 10.1155/2014/970528
[6] Ledesma J, Muñoz P, Garcia de Viedma D, Cabrero I, Loeches B, Montilla P, et al. BK virus infection in human immunodeficiency virus-infected patients. Eur J Clin Microbiol Infect Dis. 2012 Jul;31(7):1531-5. DOI: 10.1007/s10096-011-1474-9
[7] Schmidt T, Adam C, Hirsch HH, Janssen MW, Wolf M, Dirks J, et al. BK polyomavirus-specific cellular immune responses are age-dependent and strongly correlate with phases of virus replication. Am J Transplant. 2014 Jun;14(6):1334-45. DOI: 10.1111/ajt.12689
[8] Hirsch HH. Polyomavirus BK nephropathy: a (re-)emerging complication in renal transplantation. Am J Transplant. 2002 Jan;2(1):25-30. DOI: 10.1034/j.1600-6143.2002.020106.x
[9] Haby MM, Chapman E, Clark R, Barreto J, Reveiz L, Lavis JN. What are the best methodologies for rapid reviews of the research evidence for evidence-informed decision making in health policy and practice: a rapid review. Health Res Policy Syst. 2016 Nov 25;14(1):83. DOI: 10.1186/s12961-016-0155-7
[10] Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009 Jul 27;62(10):e1-34. DOI: 10.1016/j.jclinepi.2009.06.006
[11] Sánchez Fructuoso AI, Calvo N, Perez-Flores I, Valero R, Rodríguez-Sánchez B, García de Viedma D, et al. Mammalian target of rapamycin signal inhibitors could play a role in the treatment of BK polyomavirus nephritis in renal allograft recipients. Transpl Infect Dis. 2011 Dec;13(6):584-91. DOI: 10.1111/j.1399-3062.2011.00649.x
[12] Hirsch HH, Babel N, Comoli P, Friman V, Ginevri F, Jardine A, et al. European perspective on human polyomavirus infection, replication and disease in solid organ transplantation. Clin Microbiol Infect. 2014 Sep;20 Suppl 7:74-88. DOI: 10.1111/1469-0691.12538
[13] Keller N, Duquennoy S, Conrad A, Fafi-Kremer S, Morelon E, Bouvier N, et al. Clinical utility of leflunomide for BK polyomavirus associated nephropathy in kidney transplant recipients: a multicenter retrospective study. Transpl Infect Dis. 2019 Apr;21(2):e13058. Epub 2019 Mar 1. DOI: 10.1111/tid.13058
[14] Liacini A, Seamone ME, Muruve DA, Tibbles LA. Anti-BK virus mechanism of sirolimus and leflunomide alone and in combination: toward a new therapy for BK virus infection. Transplantation. 2010 Dec 27;90(12):1450-7. DOI: 10.1097/TP.0b013e3182007be2
[15] Farasati Na, Shapiro R, Vats A, Randhawa P. Effect of leflunomide and cidofovir on replication of BK virus in an in vivo culture system. Transplantation. 2005 Jan 15;79(1):116-8. DOI: 10.1097/01.tp.0000149338.97084.5f
[16] Bernhoff E, Tylden GD, Kjerpeseth LJ, Gutteberg TJ, Hirsch HH, Rinaldo CH. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J Virol. 2010 Feb;84(4):2150-6. DOI: 10.1128/JVI.01737-09
[17] Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009 Nov;9 Suppl 3:S1-155. DOI: 10.1111/j.1600-6143.2009.02834.x
[18] Schneidewind L, Neumann T, Dräger DL, Kranz J, Hakenberg OW. Leflunomide in the treatment of BK polyomavirus associated nephropathy in kidney transplanted patients – a systematic review. Transplant Rev (Orlando). 2020 Oct;34(4):100565. DOI: 10.1016/j.trre.2020.100565
[19] Hirsch HH, Randhawa PS; AST Infectious Diseases Community of Practice. BK polyomavirus in solid organ transplantation – guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019 Sep;33(9):e13528. DOI: 10.1111/ctr.13528
[20] Wojciechowski D, Chandran S, Webber A, Hirose R, Vincenti F. Mycophenolate Mofetil withdrawal with conversion to everolimus to treat BK virus infection in kidney transplant recipients. Transplantation Proc. 2017 Oct;49(8):1773-8. DOI: 10.1016/j.transproceed.2017.06.030
[21] Patel SJ, Knight RJ, Kuten SA, Graviss EA, Nguyen DT, Moore LW, et al. Ciprofloxacin for BK viremia prophylaxis in kidney transplant recipients: results of a prospective, double-blind, randomized, placebo-controlled trial. Am J Transplant. 2019 Jun;19(6):1831-7. DOI: 10.1111/ajt.15328
[22] Blyth E, Clancy L, Simms R, Gaundar S, O’Connell P, Micklethwaite K, et al. BK virus-specific T cells for use in cellular therapy show specificity to multiple antigens and polyfunctional cytokine responses. Transplantation. 2011 Nov 27;92(10):1077-84. DOI: 10.1097/TP.0b013e31823328c0
[23] Ahlenstiel-Grunow T, Sester M, Sester U, Hirsch HH, Pape L. BK polyomavirus-specific T cells as a diagnostic and prognostic marker for BK polyomavirus infections after pediatric kidney transplantation. Transplantation. 2020 Nov;104(11):2393-402. DOI: 10.1097/TP.0000000000003133
[24] Ambalathingal GR, Francis RS, Corvino D, Srihari S, Aftab BT, Smith C, et al. Proteome-wide analysis of T-cell response to BK polyomavirus in healthy virus carriers and kidney transplant recipients reveals a unique transcriptional and functional profile. Clin Transl Immunology. 2020 Jan 14;9(1):e1102. DOI: 10.1002/cti2.1102
[25] Schneidewind L, Neumann T, Krüger W, Hakenberg OW, Schmidt CA. Targeting IL-11 in the treatment of BK virus-associated haemorrhagic cystitis – a promising new approach. J Cell Mol Med. 2020;24(16):9097-100. DOI: 10.1111/jcmm.15546




