Balanitis and related inflammatory conditions affecting the penis
John N. Krieger 2
1 School of Medical Sciences, University of Sydney, Sydney, Australia
2 Section of Urology, University of Washington, Seattle, United States
Abstract
Inflammatory skin conditions of the glans (balanitis) and foreskin (posthitis) are common in uncircumcised males of all ages. Related conditions include: lichen sclerosus, psoriasis, lichen planus, schorrheic dermatitis, and Zoon (plasma cell) balanitis. These conditions often present with itching, tenderness and pain, cause significant morbidity, can be challenging to treat medically and add to the burden of health-care costs. Reducing the prevalence of penile inflammation is therefore important. In this chapter we present a systematic review of the medical literature, focusing on balanitis, balanoposthitis and lichen sclerosus. We also consider the beneficial effect of male circumcision, which, by removing the foreskin, affords substantial protection against penile inflammation. The degree of reduction in prevalence of balanitis by circumcision was found to be 68% in a meta-analysis (OR=0.32; 95% CI 0.20–0.52). Another meta-analysis showed that balanitis is associated with a 3.8-fold increased risk of penile cancer (95% CI 1.6–9.1). Yeasts and other microorganisms accumulate under the foreskin and contribute to inflammation of the surrounding penile tissue. The prevalence of penile inflammatory conditions greatly exceeds the incidence of adverse events associated with circumcision procedures of 0.4% in infancy and 1.5% or more in older males, most adverse events being minor and easily treated. Because of the potential seriousness of penile inflammation and its consequences, especially in immunocompromised, diabetic and some other patients, the high lifetime incidence of penile inflammation and the strong protection afforded by circumcision, this simple, safe procedure, best performed early in infancy, should be more widely adopted.
Key points
- Inflammatory penile skin conditions such as balanitis, balanoposthitis, lichen sclerosus and others are common, especially in uncircumcised males.
- Circumcision of males reduces balanitis by 68% and eliminates posthitis
- The benefit of circumcision in protection against these inflammatory conditions exceeds procedural risk in infancy by 20:1.
- Protection against penile skin inflammation represents an argument favoring early infant male circumcision.
1 Introduction
Inflammatory lesions of penile skin are common [1]. They include balanitis (inflammation of the glans penis), posthitis (inflammation of the foreskin) and balanoposthitis (inflammation of both the glans and foreskin) [2]. Figure 1 depicts the penile structures we refer to in this chapter. Each inflammatory lesion can be painful and associated with penile bleeding, lichen sclerosis and complications such as phimosis and paraphimosis. Affected men may be at increased risk of HIV infection if, regardless of their condition, they have sexual intercourse with an infected partner. These conditions are caused by fungal infections, most commonly the yeast Candida albicans, potentially associated with polymicrobial flora [3]. Genital yeast infection is commonly termed “candidiasis” or “thrush”. While risk is low in healthy individuals, C. albicans infection has serious consequences, including death, for millions of immunocompromised individuals, such as those infected with HIV and those being treated with immunosuppresive drugs [4]. It has been found recently that C. albicans releases a peptide toxin, candidalysin, which is a critical molecular determinant of the epithelial damage that ensues when the filamentous structures of yeast, termed hyphae, make contact with and breach the epidermal barrier of the host cell [5]. The ability of a defensive cytokine response to limit the damage caused by candidalysin is compromised in patients with HIV-related diseases, diabetes and some cancers.
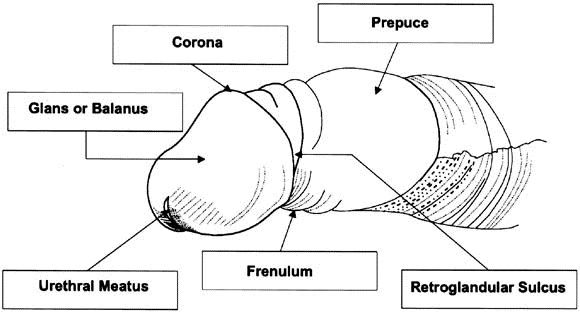
Penile inflammatory conditions can occur at any age, but are most likely to affect uncircumcised boys under 4 years of age and uncircumcised older boys and men. Males with phimosis are more likely to be affected and inflammatory penile conditions can lead to exacerbation with secondary phimosis.
Recent evidence-based policy statements recognize the protection afforded by circumcision against penile inflammatory conditions [6], [7], [8], [9]. In this chapter we discuss the various inflammatory skin conditions involving the penis and the role of circumcision in protecting against them. It was not our intention to cover penile lesions caused by sexually transmitted infections such as herpes simplex and human papillomavirus that are considered in other chapters.
2 Retrieval of reference
A search of the PubMed database on March 7, 2016 for “balanitis”, “posthitis”, “balanoposthitis”, “lichen sclerosis”, “penile inflammation” and “inflammation penis” yielded 1,081, 57, 242, 1,373, 1,342 and 1,306 publications, respectively. Searches for publications matching one or more of the keywords “circumcision”, “circumcised” or “uncircumcised” plus one or more of the keywords that appear in the previous sentence yielded: for “balanitis”, 221, 42 and 51 articles for each respective keyword combination, “posthitis” 5, 0 and 0 articles, respectively; “balanoposthitis” 42, 11 and 16 articles, respectively; “lichen sclerosis” 72, 11 and 6 articles, respectively; “penile inflammation” 241, 46 and 57 articles, respectively; and “inflammation penis” 231, 44 and 55, respectively. The title, then abstract, of each was used to judge whether an article was of sufficient quality to merit detailed review. Criteria for inclusion were that the article had to include either non-duplicated original data or a meta-analysis of original data, publication in a peer-reviewed journal, and publication in English. Major reviews were used for presenting clinical background. We retrieved the full text of every article that met our inclusion criteria for detailed review.
3 Balanitis
3.1 Clinical presentation and causes
Balanitis presents with burning, itching, swelling, pruritis, erythematous patches, and plaques or bullae involving the glans penis (Figure 2). In uncircumcised men the foreskin is often involved as well (balanoposthitis) [2]. Balanitis is worse in diabetic and immunocompromised patients, with fulminating edema or ulcers in severe cases [2].
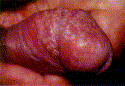
Poor hygiene is a common cause. Irritant balanitis can result from exposure to medications, such as some common antibiotics, and to allergens, including latex condoms, propylene glycol in lubricants, some spermicides and corticosteroids. Ammonia, released from urine by bacterial hydrolysis of urea, can induce inflammation of the glans and foreskin. Another common irritant responsible for balanitis is frequent washing with soaps that may contain topical allergens or irritants that may cause contact dermatitis. Various microorganisms are another cause.
3.2 Microbiology
Microorganisms under the foreskin have the potential to cause penile inflammatory conditions. The microorganisms include various bacterial species and yeasts. Much of the data come from studies in the developing world as outlined below, with only limited data from other settings. Before discussing microbiological aspects of balanitis specifically, an overview of penile microorganisms is useful.
3.2.1 Complete microbiome determined by pyrosequencing
Sophisticated 16S rRNA gene-based quantitative polymerase chain reaction (qPCR) and pyrosequencing, log response ratio, Bayseyan classification, nonmetric multidimensional scaling and permutational multivariate analysis has been used in recent years to provide a much more complete picture of the penile microbiome than traditional clinical microbiological approaches.
Key findings from a study in Rakai, Uganda using this technology found that uncircumcised men have greater microbial diversity on their coronary sulcus swabs prior to circumcision than 12 months after circumcision [10]. While abundance of aerobic bacteria did not differ significantly before and after circumcision (236 vs. 467), anaerobic species diminished (72 vs. 4.8; P<0.014) and facultative anaerobes increased (23 vs. 79; P=0.006). A further study in the same setting that used the pyrosequencing technology found significant reduction in prevalence, composition and load of anaerobic bacteria (12 taxa) at year 1 after circumcision in a randomized controlled trial [11]. The prevalence and absolute abundance of 12 anaerobic bacterial taxa decreased significantly in the men who were circumcised. It was suggested that reduction in anaerobes may account in part for the ability of circumcision to reduce HIV acquisition. Two types of aerobic bacteria became more prevalent in the men who had been circumcised. Staphylococcus species, which are skin commensals, increased after circumcision, as reported generally after circumcision.
In a subsequent study these authors concluded that the uncircumcised penis is an important niche for genital anaerobes associated with bacterial vaginosis in their female partners [12]. By qPCR and pyrosequencing bacterial vaginosis-associated taxa (including Atopobium, Megasphaera, Mobiluncus, Prevotella and Gemella) were detected in coronal sulcus specimens of both sexually experienced and inexperienced males aged 14–17 years in Indiana, USA [13]. Porphyrmonas was higher in uncircumcised men (6.4% vs. 0.3%) and Prevotella spp. were found only in uncircumcised males, in whom this organism was abundant. In contrast, Staphylococcus spp. were enriched in circumcised participants (27% vs. 5.5%) [12].
3.2.2 Conventional microbiology findings
The pyrosequencing data are roughly consistent with somewhat more limited data from studies that employed conventional clinical microbiology.
3.2.2.1 Boys
A study in India of 124 boys aged 6 weeks to 8 years swabbed prior to circumcision found E. coli, Proteus sp. and Klebsiella spp. to be the most common bacteria. After circumcision 66% lacked positive bacterial cultures [14]. A study of smegma swabs from 52 Nigerian boys aged 1 week to 11 years prior to circumcision identified 50 bacterial isolates, 58% gram-positive and 42% gram-negative, E. coli being the most common gram-negative bacterium [15]. A study in Turkey of 100 pre-pubertal boys swabbed prior to circumcision identified 72 organisms, 75% being gram-positive bacteria, 24% gram-negative bacteria and 1% Candida spp. [16]. Nine per cent had high-risk human papillomavirus (HPV) genotypes. Most bacteria were multi-drug resistant and included species capable of causing urinary tract infections. Another Turkish, study involving 78 boys aged 1 month to 14 years (mean 3.9 years), found bacterial growth in 72% before circumcision, but in only 10% after circumcision [17]. Bacterial growth was seen in all boys with phimosis, decreasing progressively to approx. 50% for increasing exposure of the glans. The most common organisms were Enterococcus (33%), Staphylococcus sp. (15%), E. coli (13%), Proteus sp. (7%) and Klebsiella sp. (3%).
Fungi (exclusively C. albicans) were found in 3.5% of 200 Iranian infants prior to circumcision [18]. The low incidence of fungal colonization led the authors to advocate circumcision. In boys aged 8 months to 18 years (mean 6.4 years) the incidence of fungi in those not circumcised was 44% compared with 18% in circumcised boys, i.e., was 2.4 times higher in the uncircumcised [19]. The fungal species found were, in order of frequency, Malassezia globosa, M. furfur, M. slooffiae, C. albicans, C. tropicalis and C. parapsilosis. In infancy all were present in the uncircumcised, but none were found in the circumcised. A gradual accumulation then occurred in a portion of the circumcised boys with age so that by age 18 fungi were found in 37.5%, being much lower than the prevalence of 62.5% seen uncircumcised boys. The various studies above involved traditional methods for identification of microorganism species. Bteer methods, such as pyrosequencing referred to above, are needed to confirm the findings.
3.2.2.2 Men
A study in India of 350 men found those who were uncircumcised were more likely to harbor bacterial pathogens in the coronal sulcus [20]. Gram-positives, gram-negatives and any pathogen were 1.9-, 2.4- and 2.8-times higher in uncircumcised men.
C. albicans is the most frequent fungal isolate from the penis [21]. Fungi are normal flora that can present as overgrowth under certain conditions, especially in diabetic patients with phimosis. Candida colonization was seen in 16% of men visiting a STI clinic in Coventry, UK [22]. Smegma, produced under the foreskin, consists of 27% fat and 13% protein, and contributes to the higher occurrence of Malassezia spp. in the uncircumcised (49% vs. 7%) [23]. Circumcision reduced the frequency of yeast colonization from 11% to 1.3% (P<0.008) [24].
Circumcision of men reduces the risk of bacterial vaginosis in their female partners [25]. Further work in Rakai, Uganda, by these authors found that circumcision was associated with a significant decrease in anaerobic genera associated with bacterial vaginosis, specifically Anaerococcus spp., Finegoldia spp., Peptoniphilus spp. and Prevotella spp. [10]. Bacteria responsible for bacterial vaginosis are exchanged between partners during sexual intercourse [12].
3.2.3 Microbiology of balanitis
Mycotic infections are a common cause of balanitis. Candida species are the most prevalent, being found in approximately one-third of cases. While carriage of yeasts on the penis is common, symptomatic infection is seen more often in uncircumcised males [3]. Patients present with mild burning and pruritis. Clinical features include mild glazed erythrema, satellite eroded pustules and moist curd-like accumulations [2]. Bacterial superinfection with streptococci or staphylococci increases pain.
Bacteria by themselves are the second most common cause of infectious balanitis, Streptococcus spp. being seen most often. Less common are: Hemophilus parainfluenza, Klebsiella sp., Staphylococcus epidermidis, Enterococcus, Proteus sp., Morganella sp. and Escherichia coli [2].
Chlamydia trachomatis, genital mycoplasmas and bacterial sexually transmitted infections (STIs) such as Neisseria gonorrhoeae, Haemophiluis ducreyi and others can be associated with balanitis and balanoposthitis [2]. N. gonorrhoeae produces an endotoxin that may be responsible for edema and erythrema of the foreskin [26]. Gardnerella vaginalis is responsible for symptomatic anaerobic-related balanitis in men that has the additional presentation of a subpreputial “fishy”-smelling discharge similar to the odor associated with bacterial vaginosis in women [2]. The prevalence of this STI was 15% and 25% among heterosexual STI clinic attendees in London [27] and Alabama [28], respectively [27].Other causes of balanitis and balanoposthitis include viral STIs such as high-risk HPV types and parasitic infections such as Trichomonas vaginalis and protozoa, all seen mostly in uncircumcised men [2].
3.3 Balanitis in boys
Balanitis affects approximately 4% of boys, most commonly during the pre-school years [29]. Traditionally, it has been said that balanitis in babies is caused by soiled diapers and playing or sitting in dirty areas, although we could find no strong data supporting these statements. Antibiotic therapy can favour over-growth of yeasts as well other microorganisms, which in turn can favor development of balanitis. In fact, any factor that causes a substantial increase in microorganisms has the potential to contribute to development balanitis in boys.
Lack of circumcision is a predisposing factor, especially when the foreskin is partly or completely non-retractable [29]. It has been said that the most obvious medical reasons for circumcision of a boy is to protect him against balanitis and posthitis [3]. In boys the incidence of balanitis was reported as being twice as high in those who were not circumcised [30], [31], [32], [33]. Cases of balanitis caused by the group A or B hemolytic Streptococcus spp. have been reported in prepubertal [34] and postpubertal [35] uncircumcised boys, respectively. Newer, non-culture, techniques, such as pyrosequencing, are needed to confirm and expand on such fidnings.
Balanitis is especially common in uncircumcised boys with phimosis compared with those without phimosis: 25% vs. 6%, respectively, for ages less than 5 years and 24% vs. 12% for males older than 5 years [36]. In that study ballooning was also more common in uncircumcised boys suffering from phimosis (34% vs. 2% and 20% vs. 4% for the respective age groups) [36].
3.4 Balanitis in men
Apart from exposure to certain medications, allergens and chemical irritants, lack of circumcision has been consistently associated with balanitis in men. For example, balanitis was reported in 11–13% of uncircumcised men, but in only 2% of circumcised men [32], [33]. In men aged 16–95 years (mean age 47 years), a 3-year prospective review at a multi-specialty penile dermatology clinic in Edinburgh, UK, diagnosed non-specific balanitis in 22% of patients [37]. Of the episodes where circumcision status was documented, 18% were circumcised and 53% were uncircumcised. Prevalence of balanitis was 11% in men (all uncircumcised) attending an STI clinic in Portugal [38]. In a large randomized controlled trial involving young men, over the 18 months of follow-up, balanitis was seen in 0.7% of the uncircumcised men, but in none of the men who received circumcision [39].
Uncircumcised diabetic men have a high prevalence of symptomatic balanitis of 35% [32], [33]. Of men with acquired phimosis, 26% had a history of diabetes [40]. Phimosis increases the risk of infection of the foreskin and glans. Over the period 1942–1945 during World War II there were 146,000 hospitalizations of US troops for balanitis, balanoposthitis, phimosis and paraphimosis [41]. It was remarked that, “the man-hours lost as a result of circumcisions and adjuvant therapy were costly to the war effort and exasperated the commanding officers. Time and money could have been saved had prophylactic circumcision been performed before the men were shipped overseas” [41].
3.5 Meta-analysis of balanitis and circumcision status
As apparent from the presentation above, circumcision provides substantial protection against balanitis [42]. A meta-analysis of relevant studies [30], [31], [32], [39], [42], [43], [44], [45] found that the prevalence of balanitis was 68% lower in circumcised vs. uncircumcised males (odds ratio = 0.32; 95% CI 0.20–0.52) [46] (Figure 3). Thus, inflammatory dermatoses of the penis are 3.2 times (95% CI 1.9–5.0) higher in uncircumcised males.
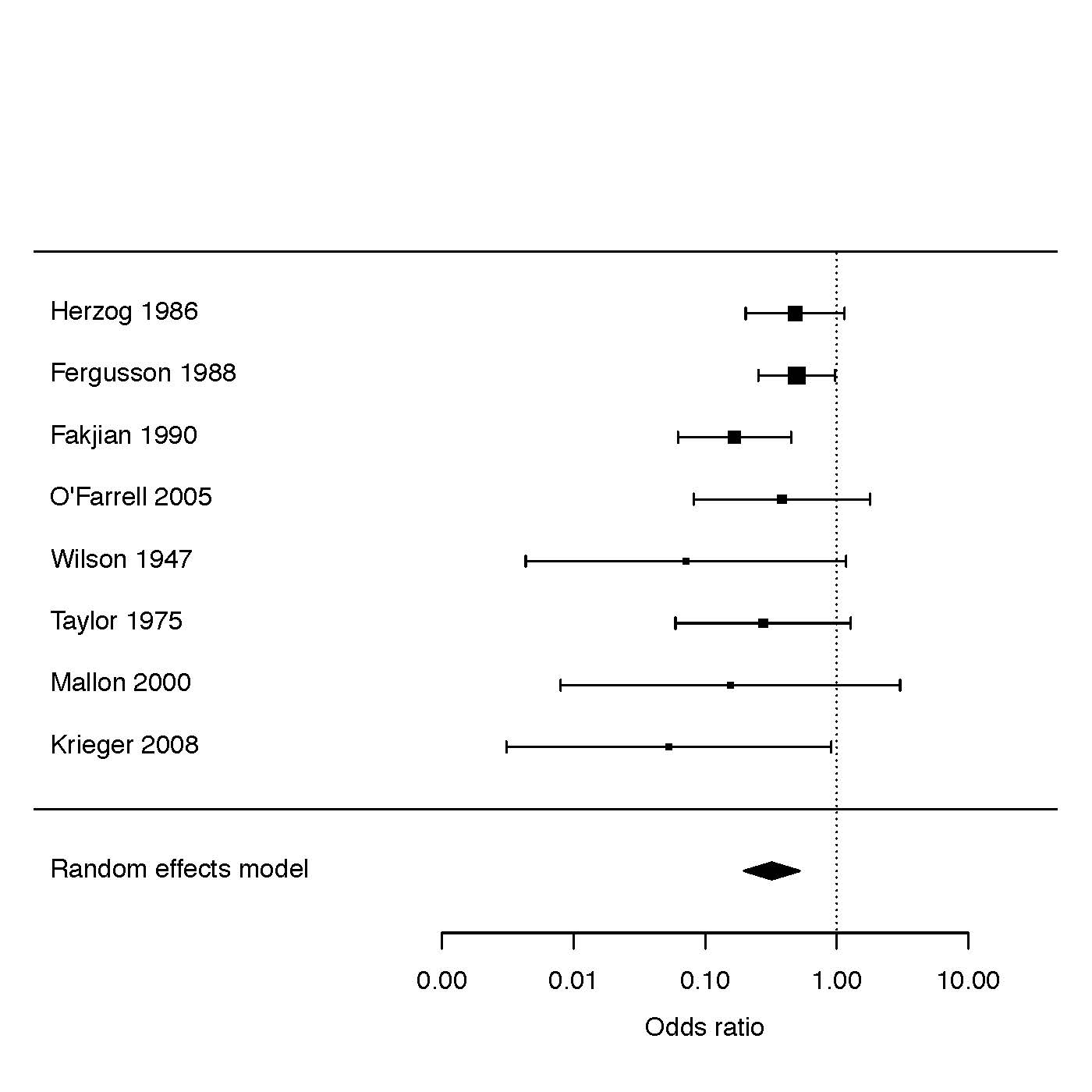
Shown are results of a Forest plot after analysis of data from 8 studies [30], [31], [32], [39], [42], [43], [44], [45].
Since inclusion of an anomalous outlier study [119] led to significant between-study heterogeneity (P=0.03),
but when excluded no significant heterogeneity remained (P=0.40), the meta-analysis that was performed
did not include data from that study.
3.6 Treatment
Topical therapy using a cream or ointment is effective in treatment of balanitis, but recurrence is frequent especially in patients with risk factors such as phimosis or diabetes. Topical anti-fungals, if applied consistently until symptoms disappear, may also be effective in treatment of sexually acquired balanitis; treatment of the partner is also important in order to reduce the risk of relapse [47]. Circumcision would appear desirable to reduce the occurrence of balanitis.
4 Balanoposthitis
Balanoposthitis only occurs in uncircumcised males. Its prevalence is generally lower than balanitis [29]. The entire distal end of the penis (foreskin and glans) presents as red, painful and swollen, and is often accompanied by a foul smelling, purulent discharge [48]. Balanophosthitis can lead to a vicious cycle. After each infection, the foreskin will heal by fibrosis, in which there is thickening and scarring of connective tissue, and this will further shrink the tight foreskin. The symptoms of balanoposthitis represent a strong medical indication for circumcision.
4.1 Balanoposthitis in boys
The most common ages for balanoposthitis to present in childhood are between 2 and 5 years [29], which contradicts claims of soiled diapers, etc. referred to above. In young boys balanoposthitis tends to be associated with phimosis and inability to clean under the foreskin because the foreskin is still lightly attached to the penis beneath it [48].
4.2 Balanoposthitis in men
Balanoposthitis was found in 20% of 194 [49] consecutive unselected UK men, all of whom were uncircumcised [49]. A Brazilian study of men presenting for prostate cancer screening found balanoposthitis in 12% [50]. Prevalence was 58% higher in those with a history of non-specific urethritis [50]. Balanoposthitis is especially common in uncircumcised diabetic men [32], [50], [51]. A dysfunctioning, shrunken penis may be a contributing factor [32]. Not surprisingly, balanoposthitis in diabetic men adds to their frequent diabetic neuropathy and peripheral vascular disease, resulting in frequent sexual dysfunction. Diabetes is common, inherited and rising in incidence, suggesting that a family history of diabetes, should be considered when deciding whether to circumcise an infant at birth.
4.3 Treatment
While use of local hygienic measures has been suggested for treatment of nonspecific balanoposthitis, if the condition is recalcitrant, use of anti-fungal and antibiotic creams has been suggested [52]. Circumcision is recommended as the definitive cure [52], [47]. Of 476 boys who were circumcised beyond the neonatal period, balanoposthitis was the reason in 23% [53].
5 Other penile skin conditions
Various other penile skin diseases have been described. These disorders include: psoriasis, penile infections, lichen sclerosus (detailed in next section), lichen planus, schorrheic dermatitis, and Zoon (plasma cell) balanitis (described in extensive reviews of diseases of the penis: [2], [33], [54]). These conditions are either much more common in, or totally confined to, uncircumcised males. In all, 34 different conditions, including these, and their frequency, were diagnosed in 226 men over 3 years in a clinic in Edinburgh [37]. Penile dermatoses in general have been reported in twenty [55], five [56], three [37] and two [42] times as many uncircumcised men as men who were circumcised. Below, we summarize data on several of the most prominent conditions.
In one large series, all patients with Zoon balanitis, Bowenoid papulosis, and nonspecific balanoposthitis were uncircumcised [42]. Bowenoid papulosis is a condition that occurs mainly in young sexually active men [54]. A single case of Zoon balanitis in a circumcised man has, however, been described [57]. Typical symptoms of Zoon balanitis include erythrema (in 100%), swelling (in 91%), discharge (in 73%), dysuria (in 13%), bleeding (in 2%) and ulceration (in 1%) [33]. Mycobacterium smegmatis has been implicated in Zoon balanitis [2]. In a study of Zoon balanitis involving 112 uncircumcised men aged 24–70 years, lesions were present on the foreskin and glans of 59%, foreskin only in 23% and glans only in 18%. These observations led the authors to conclude, “The importance of circumcision as the treatment of choice is emphasized” [58]. Improvement in the lesions associated with Zoon balanitis has, however, been achieved with 0.1% tacrolimus ointment [59]. Erosive lichen planus is associated with increased mast cells, foreskin scarring and phimosis in uncircumcised men [60].
6 Lichen sclerosus
6.1 Definition
Lichen sclerosus (formally termed either lichen sclerosus et atrophicus or balanitis xerotica obliterans: BXO) is a chronic, progressive, sclerosing inflammatory anogenital skin disease of uncertain cause [2]. The characteristic clinical appearance is shown in Figure 4 [61]. It is amongst the most serious inflammatory conditions affecting the penis. As a result the literature on lichen sclerosus is considerable.
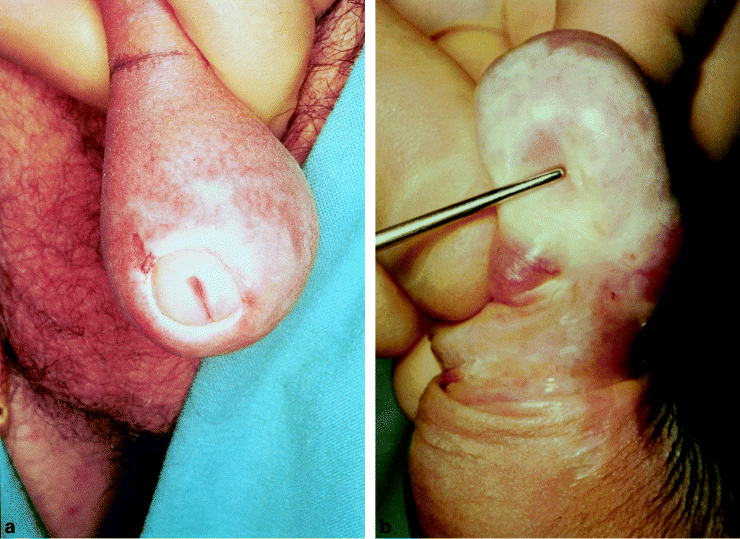
b) Meatal structure which can result [61]
6.2 Clinical presentation
Lichen sclerosus has been described as, “an urologist’s nightmare” [62]. It presents as single or multiple erythematous papules, macules, or plaques that progress to sclerotic or atrophic white, ivory or blue-white coalescent flat-topped papules and plaques [2]. Lesions commonly involve the glans and foreskin, although the frenulum, urethral meatus and fossa navicularis may be involved as well. A sclerotic white ring at the tip of the foreskin is diagnostic of lichen sclerosus. Shaft and perianal involvement is rare. Serrous and hemorrhagic bullae, erosions, fissures, telangiectasia and petechiae of the glans can occur as well. The foreskin may be adherent to the glans and as the disease progresses the coronal sulcus and frenulum may be obliterated and the meatus gradually narrows. More than 10 years is required for the disease to progress through the entire urethra [63]. The latter may result in significant urinary retention accompanied by subsequent retrograde damage to the posterior urethra, bladder and kidneys [2]. Eventual sloughing of the distal 0.5 cm of the urethra may occur.
Lichen sclerosus can present at any age and is regarded as common [64]. Prevalence has been estimated as 1-in-300 to 1-in-1,000 [65]. Lichen sclerosus prevalence in prepubertal German boys was 0.1–0.4% [66] and in Danish boys aged 1–17 years was 0.37% [67].
Penile lichen sclerosus regarded as common in middle-aged uncircumcised men. In boys, penile lichen sclerosus is a common cause of phimosis [68], [69]. Early in its course lichen sclerosus is often asymptomatic. Men may complain of phimosis, pruritis, burning, hypoesthesia of the glans, dysuria, urethritis with or without discharge, painful erections and sexual dysfunction [2]. In a Swedish study 56% of lichen sclerosus patients complained of an adverse effect on their sex lives [70].
Figure 5 shows the penile sites affected by lichen sclerosus in a study of 66 men at a genitourinary clinic in Oxford, UK [64]. The frequency of these was: meatus (64%; 37% of these having meatal narrowing), foreskin (55%), shaft and glans (20%) [64]. Thirty per cent of the men in this study did not complain of symptoms related to lichen sclerosus at the time of diagnosis. Nine per cent had had a circumcision. A 2014 review of 40 reports found that lichen sclerosus affected the foreskin and glans in 57–100% of cases, the meatus in 4–37% and urethra in 20% [71]. These studies found that disease progression may lead to phimosis and severe urethral stricture disease [71]. Lichen sclerosus prevalence has generally been thought to peak in the fourth decade of life in men [72], although others reported it peaks in the third decade [73]. Biopsy of foreskins following circumcision diagnosed lichen sclerosus histologically in 4–19% [2], [33]. The narrow foreskin opening causes urinary obstruction that can be partial or complete. As well as urethral stenosis, meatal stenosis is seen, making lichen sclerosus a significant medical condition in men [74], [75].
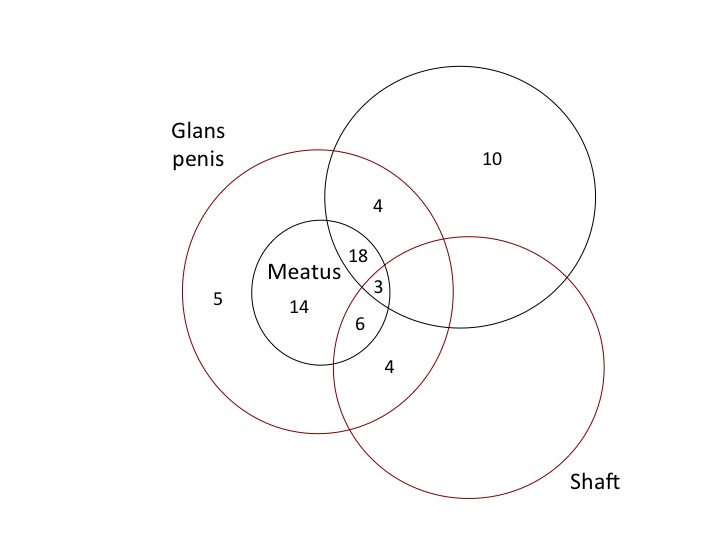
of each in a study of 66 cases in the UK [64]
6.3 Etiology of lichen sclerosus
Lichen sclerosus is presumed to be of autoimmune origin, exacerbated by the warm and moist local environment under the foreskin [66]. Genetic and hormonal factors may contribute [76]. Another factor can be the isomorphic response [2]. Of patients with lichen sclerosus, 98% were uncircumcised [42]. Late circumcision (i.e., after age 13 years) has also been invoked [77].
6.4 Lichen sclerosus in boys
Once thought to be rare, and a disorder presenting in adulthood, lichen sclerosus is now regarded as common in young boys [74], [78], [79]. Average age of diagnosis in boys is 9–11 years [80]. Atopic skin diathesis is seen in 25% of cases in boys and is thought to predispose to lichen sclerosus [81]. Thus, whereas a rate of 1% has been reported [82], two UK studies found the prevalence of lichen sclerosus to be 5% and 6% in uncircumcised boys under 18 years and under 15 years of age, respectively [83], [84]. In older studies, histological examination of foreskins removed for various reasons revealed lichen sclerosus in 3.6–19% [85], [86], [87], [88], [89], [90]. A 2007 study in Plymouth, UK, of 422 boys aged 3 months to 16 years (mean 6 years) referred to a pediatric general surgical outpatient department with foreskin problems diagnosed 55.9% as being normal and the remainder (44.1%) underwent surgery, 35% by circumcision, 8% preputial adhesiolysis and 0.1% frenuloplasty [74]. Histological abnormalities were seen in 85% of the foreskins removed by circumcision and chronic inflammation was seen in 47%, lichen sclerosis in 35%, fibrosis in 3%, while 13% were histologically normal [74].
A recent systematic review evaluated the global prevalence of lichen sclerosus in 13 studies. The overall prevalence was 35% in foreskins from boys circumcised for any reason [91]. Lichen sclerosus was regarded as an indication for circumcision [74]. A prospective study in Budapest involving 1,178 boys who presented consecutively over the decade 1991–2001 and who underwent circumcision, identified lichen sclerosus in 40% of histological specimens with a peak prevalence of 76% among those aged 9–11 years [92]. In this study, 19% of boys had an early, 60% had an intermediate and 21% had a late form of lichen sclerosus.
Boys with phimosis often have lichen sclerosus [93]. Prevalence of lichen sclerosus in acquired phimosis cases ranges from 10% [94] to 80–90% [66] in more recent studies.
Lichen sclerosus can be the cause of pathological phimosis, which arises from secondary cicatrization of the foreskin orifice. In one study, lichen sclerosus was felt to be responsible for secondary phimosis in all pediatric patients who underwent circumcision [92]. In another study 37% of pediatric patients with severe phimosis had lichen sclerosus [95]. A different study found lichen sclerosus in 60% of boys with acquired phimosis and in 30% of those with congenital phimosis [93]. Even more of the boys presented with inflammatory features of the foreskin (88% and 82%, respectively). The study also examined boys with congenital hypospadias, 61% showing symptoms of inflammation and 15% had features consistent with lichen sclerosus. In one series from Boston, of 41 pediatric patients with lichen sclerosus, 52% had been referred for phimosis, 13% for balanitis and 10% for buried penis [96].
6.5 Lichen sclerosus in men
In an Italian study of men (mean age 46 years), 85% of biopsies were positive for lichen sclerosus, lichen sclerosus of the foreskin being documented by histology in 93% of cases, of the meatus in 92% of cases, of the fossa navicularis in 84% and of the penile urethra in 71% [63]. A study of Hungarian men circumcised for phimosis documented lichen sclerosus in 62% [97].
In older patients, progressive lichen sclerosus or other inflammatory changes can lead to phimosis [98]. In elderly men, lichen sclerosus with phimosis can also cause lower urinary tract symptoms [99]. Phimosis in older men is, moreover, associated with 44–85% of cases of penile cancer [71], [100]. In men not circumcised in childhood phimosis was strongly associated with development of invasive penile cancer, as was high-risk human papillomavirus [101]. Oncogenic HPV was seen in 23% of 92 Italian men with lichen sclerosus (mean age 68 years) compared to 15% of men without lichen sclerosus (mean age 57 years), suggesting that lichen sclerosus leads to slower clearance of HPV [102]. Amongst 226 males aged 16–95 years attending a penile dermatology clinic in Edinburgh, penile intraepithelia neoplasia was diagnosed in 6% and invasive penile cancer in 2% [37]. Penile cancer was found in 1% of 771 Swedish men aged 48.6 years (range 22–92) diagnosed with lichen sclerosus during 1997–2007 [70]. Another study found penile cancer in 4–8% of men with lichen sclerosus [103].
A random-effects meta-analysis of 8 studies found phimosis to be associated with a 12-fold (95% CI 5.6–26) increase in penile cancer risk [104]. Since lifetime prevalence of penile cancer is approximately 1 in 1,000 [6], [7], [104], it is clear that lichen sclerosus represents an important, potentially preventable, risk factor for penile cancer.
6.6 Treatment of lichen sclerosus
6.6.1 Treatment in men
A recent review pointed out that circumcision is curative in “nearly 100%” of cases [105]. One study gave a cure rate of over 75% [72] and another stated that when confined to the foreskin, circumcision results in a long-term cure in 92% of lichen sclerosus patients [61].
In contrast, steroid creams limit disease progression but do not cure many cases [72], [106]. Specifically, it has been claimed that steroids lead to an improvement in 41–76% and a cure in only 50–60% of cases in men [72]. In the second author’s experience steroids are not as effective as this. Recurrence of lichen sclerosus after steroid treatment may occur after 5 years [105]. In a Swedish study 30% of men reported that the outcome of local steroid treatment was “good”, while 37% said it was “medium” and 16 “poor” (17% did not answer) [70]. All men aged 20–45 with lichen sclerosus in one Indian report were circumcised in preference to use of steroid creams [107]. In another study in India, 77% received circumcision for treatment [62].
Once progression to urethral involvement has occurred, treatment becomes much more difficult [71]. It may include meatotomy or meatoplasty for meatal stenosis and urethroplasty for the urethra. When extensive disease affects the full length of the urethra, perineal urethrostomy may be required [71]. Treatment in an Italian study of men of mean age 46 years included circumcision, meatotomy, navicularis uroplasty, extensive grafting procedures and perineal urethrostomy [63]. Mean time between diagnosis of lichen sclerosus and development of penile cancer is 12 years [71].
6.6.2 Treatment in boys
Circumcision was recommended as the treatment of choice for lichen sclerosus in boys [61], [92], [94], whereas conservative treatment with topical steroids was considered as “controversial” [66], [81]. Steroids have significant side effects and should be avoided in children [71]. Preputioplasty of boys for lichen sclerosus is regarded by some as effective, although 13% developed recurrent symptoms [108]. Preputioplasty or frenuloplasty are never a first choice and are an option when paretns do not agree to circumcision. In a report from Boston, 46% of pediatric lichen patients underwent curative circumcision; 27% also had lichen sclerosus involvement of the meatus and received not only circumcision, but meatotomy or meatoplasty [96]. In all, 22% required extensive plastic surgery of the penis, including buccal mucosa grafts, demonstrating a more severe and morbid clinical course. A study in Liverpool, UK, of 300 boys circumcised after a clinical diagnosis of lichen sclerosus, lichen sclerosus was confirmed by histology in 80% (mean age 9 years; range 4–16 years) and 1 in 5 required subsequent meatal dilatation or meatotomy for meatal pathology [109].
7 Risks from circumcision and lack of circumcision in the newborn period
The benefits of male circumcision in reducing the prevalence and incidence of penile inflammation and other adverse medical conditions have to be weighed against the risks of adverse events arising from the circumcision procedures. A 1989 report found that adverse events occurred after 0.19% of 100,157 circumcisions performed in the first month of life [110]. Most adverse events were minor and easily treatable, leading to complete resolution. In contrast, complications were seen in 0.24% of 35,929 uncircumcised infants in that study. All complications in uncircumcised infants were related to UTI and included 32 infants who developed concomitant bacteremia, 3 with meningitis, 2 with renal failure and 2 who died [110].
Subsequent studies reported similar findings. The most recent and authoritative data came from a study in 2014 by the Centers for Disease Control and Prevention. This study documented adverse events associated with circumcision of 1.4 million males in the USA, including 93.3% circumcised as newborns [111]. The prevalence of adverse events after newborn circumcision was 0.4%. Virtually all of these adverse events were minor, easily treatable and after treatment there was complete resolution. Adverse events were 20-fold higher for circumcision of boys aged 1–9 years and 10-fold higher for males older than 10 years old [111].
8 Further research
The compelling evidence for benefits of male circumcision in protection against penile inflammatory conditions raises several issues for further research:
- Developing methods to improve education of medical professionals and the general public on the long-term benefits of medical male circumcision early in infancy for protection against penile inflammatory conditions.
- Further definition of the magnitude and mechanisms underlying the reduction of risk of balanitis, balanoposthitis, lichen sclerosus and various rarer penile inflammatory conditions as a consequence of male circumcision.
- Improving implementation of medical male circumcision as a public health measure.
9 Conclusions
Balanitis and balanoposthitis are common. Not only do they lead to frequent medical consultations, if not treated, the consequences can include acquired phimosis and lichen sclerosis, the treatment of which is challenging. Men with penile inflammation are also at increased risk of HIV infection if they engage in sexual intercourse. While topical anti-fungal creams can be used to treat each of these, usually accompanied by advice on hygiene, the definitive treatment is circumcision. Based on the evidence, circumcision of males, particularly early in life, substantially reduced the risk of penile inflammatory conditions [112]. Protection in infancy against penile inflammation was emphasized in the 2012 American Academy of Pediatrics infant male circumcision policy recommendations [6], the draft recommendations on male circumcision by the US Centers for Disease Control and Prevention [7], the American Urological Association policy on circumcision [8] and the infant male circumcision policy statement of the Circumcision Academy of Australia [9]. Coupled with its other lifetime benefits [6], [7], [8], [113], circumcision of all infant males would seem desirable from a public health perspective.
As pointed out by the US Centers for Disease Control and Prevention [8], benefits of male circumcision exceed risks by 100 to 1 [113]. Early infant circumcision is as protective against adverse medical conditions as are many vaccines given to children to prevent other infections and diseases [114]. For example the level of protection deemed acceptable against influenza vaccines [115], [116] justifies claims that circumcision in infancy be regarded as a “surgical vaccine” [48], [117], [118]. Even more so when one considers that over their lifetime half of uncircumcised males will suffer a medical condition caused by the retention of the foreskin [113].
References
[1] West DS, Papalas JA, Selim MA, Vollmer RT. Dermatopathology of the foreskin: an institutional experience of over 400 cases. J Cutan Pathol. 2013 Jan;40(1):11-8. DOI: 10.1111/cup.12032[2] English MJC III, Laws CRA, Keough CGC, Wilde CJL, Foley LJP, Elston LDM. Dermatoses of the glans penis and prepuce. J Am Acad Dermatol. 1997 Jul;37(1):1–26. DOI: 10.1016/S0190-9622(97)70207-X
[3] Edwards S. Balanitis and balanoposthitis: a review. Genitourin Med. 1996 Jun 1;72(3):155–9. DOI: 10.1136/sti.72.3.155
[4] Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden Killers: Human Fungal Infections. Sci Transl Med. 2012 Dec 19;4(165):165rv13-165rv13. DOI: 10.1126/scitranslmed.3004404
[5] Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Höfs S, Gratacap RL, Robbins J, Runglall M, Murciano C, Blagojevic M, Thavaraj S, Förster TM, Hebecker B, Kasper L, Vizcay G, Iancu SI, Kichik N, Häder A, Kurzai O, Luo T, Krüger T, Kniemeyer O, Cota E, Bader O, Wheeler RT, Gutsmann T, Hube B, Naglik JR. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016 Apr;532(7597):64-8. DOI: 10.1038/nature17625
[6] American Academy of Pediatrics Task Force on Circumcision. Male circumcision. Pediatrics. 2012 Sep;130(3):e756-85. DOI: 10.1542/peds.2012-1990
[7] Department of health and human services; Centers for Disease Control and Prevention (CDC). Recommendations for Providers Counseling Male Patients and Parents Regarding Male Circumcision and the Prevention of HIV Infection, STIs, and Other Health Outcomes: Docket No. CDC–2014–0012. Federal Register. 2014 Dec 2 [cited 2015 Jan 5];79(231). Available from: https://www.regulations.gov/document?D=CDC-2014-0012-0001
[8] American Urological Association. Circumcision. 2012 [cited 2015 Mar 10]. Available from: http://www.auanet.org/guidelines/circumcision
[9] Morris BJ, Wodak AD, Mindel A, Schrieber L, Duggan KA, Dilly A, Willcourt RJ, Cooper DA, Lumbers ER, Russell CT, Leeder SR. Infant male circumcision: An evidence-based policy statement. Open J Prevent Med. 2012;2(1):79–92. DOI: 10.4236/ojpm.2012.21012
[10] Price LB, Liu CM, Johnson KE, Aziz M, Lau MK, Bowers J, Ravel J, Keim PS, Serwadda D, Wawer MJ, Gray RH. The effects of circumcision on the penis microbiome. PLoS One. 2010 Jan 6;5(1):e8422. DOI: 10.1371/journal.pone.0008422
[11] Liu CM, Hungate BA, Tobian AA, Serwadda D, Ravel J, Lester R, Kigozi G, Aziz M, Galiwango RM, Nalugoda F, Contente-Cuomo TL, Wawer MJ, Keim P, Gray RH, Price LB. Male circumcision significantly reduces prevalence and load of genital anaerobic bacteria. MBio. 2013 Apr;4(2):e00076. DOI: 10.1128/mBio.00076-13
[12] Liu CM, Hungate BA, Tobian AA, Ravel J, Prodger JL, Serwadda D, Kigozi G, Galiwango RM, Nalugoda F, Keim P, Wawer MJ, Price LB, Gray RH. Penile Microbiota and Female Partner Bacterial Vaginosis in Rakai, Uganda. MBio. 2015 Jun;6(3):e00589. DOI: 10.1128/mBio.00589-15
[13] Nelson DE, Dong Q, Van der Pol B, Toh E, Fan B, Katz BP, Mi D, Rong R, Weinstock GM, Sodergren E, Fortenberry JD. Bacterial communities of the coronal sulcus and distal urethra of adolescent males. PLoS ONE. 2012;7(5):e36298. DOI: 10.1371/journal.pone.0036298
[14] Laway MA, Wani ML, Patnaik R, Kakru D, Ismail S, Shera AH, Shiekh KA. Does circumcision alter the periurethral uropathogenic bacterial flora. Afr J Paediatr Surg. 2012 May-Aug;9(2):109-12. DOI: 10.4103/0189-6725.99394
[15] Anyanwu LJ, Kashibu E, Edwin CP, Mohammad AM. Microbiology of smegma in boys in Kano, Nigeria. J Surg Res. 2012 Mar;173(1):21-5. DOI: 10.1016/j.jss.2011.04.057
[16] Balci M, Tuncel A, Baran I, Guzel O, Keten T, Aksu N, Atan A. High-risk Oncogenic Human Papilloma Virus Infection of the Foreskin and Microbiology of Smegma in Prepubertal Boys. Urology. 2015 Aug;86(2):368-72. DOI: 10.1016/j.urology.2015.04.034
[17] Tarhan H, Akarken I, Koca O, Ozgü I, Zorlu F. Effect of preputial type on bacterial colonization and wound healing in boys undergoing circumcision. Korean J Urol. 2012 Jun;53(6):431-4. DOI: 10.4111/kju.2012.53.6.431
[18] Mousavi S A, Shokohi T, Hedayati M T, Mosayebi E, Abdollahi A, Didehdar M. Prevalence of Yeast Colonization on Prepuce of Uncircumcised Children. J Mazandaran Univ Med Sci. 2015;25(128):118-122.
[19] Iskit S, Ilkit M, Turç-Biçer A, Demirhindi H, Türker M. Effect of circumcision on genital colonization of Malassezia spp. in a pediatric population. Med Mycol. 2006 Mar;44(2):113-7.
[20] Schneider JA, Vadivelu S, Liao C, Kandukuri SR, Trikamji BV, Chang E, Antonopoulos D, Prasad S, Lakshmi V. Increased Likelihood of Bacterial Pathogens in the Coronal Sulcus and Urethra of Uncircumcised Men in a Diverse Group of HIV Infected and Uninfected Patients in India. J Glob Infect Dis. 2012 Jan;4(1):6-9. DOI: 10.4103/0974-777X.93750
[21] Aridogan IA, Izol V, Ilkit M. Superficial fungal infections of the male genitalia: a review. Crit Rev Microbiol. 2011 Aug;37(3):237-44. DOI: 10.3109/1040841X.2011.572862
[22] David LM, Walzman M, Rajamanoharan S. Genital colonisation and infection with candida in heterosexual and homosexual males. Genitourin Med. 1997 Oct 1;73(5):394–6. DOI: 10.1136/sti.73.5.394
[23] Mayser P, Schütz M, Schuppe HC, Jung A, Schill WB. Frequency and spectrum of Malassezia yeasts in the area of the prepuce and glans penis. BJU Int. 2001 Oct;88(6):554-8.
[24] Aridogan IA, Ilkit M, Izol V, Ates A, Demirhindi H. Glans penis and prepuce colonisation of yeast fungi in a paediatric population: pre- and postcircumcision results. Mycoses. 2009 Jan;52(1):49-52. DOI: 10.1111/j.1439-0507.2008.01535.x
[25] Gray RH, Kigozi G, Serwadda D, Makumbi F, Nalugoda F, Watya S, Moulton L, Chen MZ, Sewankambo NK, Kiwanuka N, Sempijja V, Lutalo T, Kagayii J, Wabwire-Mangen F, Ridzon R, Bacon M, Wawer MJ. The effects of male circumcision on female partners' genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009 Jan;200(1):42.e1-7. DOI: 10.1016/j.ajog.2008.07.069
[26] Fiumara NJ, Kahn S. Contact dermatitis from a gonococcal discharge: a case report. Sex Transm Dis. 1982 Jan-Mar;9(1):41-2.
[27] Dawson SG, Ison CA, Csonka G, Easmon CSF. Male carriage of Gardnerella vaginalis. Br J Vener Dis. 1982 Aug 1;58(4):243–5. DOI: 10.1136/sti.58.4.243
[28] Schwebke JR, Rivers C, Lee J. Prevalence of Gardnerella vaginalis in male sexual partners of women with and without bacterial vaginosis. Sex Transm Dis. 2009 Feb;36(2):92-4. DOI: 10.1097/OLQ.0b013e3181886727
[29] Escala JM, Rickwood AM. Balanitis. Br J Urol. 1989 Feb;63(2):196-7.
[30] Herzog LW, Alvarez SR. The frequency of foreskin problems in uncircumcised children. Am J Dis Child. 1986;140(3):254-256. DOI: 10.1001/archpedi.1986.02140170080036
[31] Fergusson DM, Lawton JM, Shannon FT. Neonatal circumcision and penile problems: an 8-year longitudinal study. Pediatrics. 1988 Apr;81(4):537-41.
[32] Fakjian N, Hunter S, Cole GW, Miller J. An argument for circumcision. Prevention of balanitis in the adult. Arch Dermatol. 1990 Aug;126(8):1046-7.
[33] Köhn FM, Pflieger-Bruss S, Schill WB. Penile skin diseases. Andrologia. 1999;31 Suppl 1:3-11.
[34] Orden B, Martin R, Franco A, Ibañez G, Mendez E. Balanitis caused by group A beta-hemolytic streptococci. Pediatr Infect Dis J. 1996 Oct;15(10):920-1.
[35] Lucks DA, Venezio FR, Lakin CM. Balanitis caused by group B streptococcus. J Urol. 1986 May;135(5):1015.
[36] Ladenhauf HN, Ardelean MA, Schimke C, Yankovic F, Schimpl G. Reduced bacterial colonisation of the glans penis after male circumcision in children--a prospective study. J Pediatr Urol. 2013 Dec;9(6 Pt B):1137-44. DOI: 10.1016/j.jpurol.2013.04.011
[37] Pearce J, Fernando I. The value of a multi-specialty service, including genitourinary medicine, dermatology and urology input, in the management of male genital dermatoses. Int J STD AIDS. 2015 Sep;26(10):716-22. DOI: 10.1177/0956462414552695
[38] Lisboa C, Ferreira A, Resende C, Rodrigues AG. Infectious balanoposthitis: management, clinical and laboratory features. Int J Dermatol. 2009 Feb;48(2):121-4. DOI: 10.1111/j.1365-4632.2009.03966.x
[39] Krieger JN, Mehta SD, Bailey RC, Agot K, Ndinya-Achola JO, Parker C, Moses S. Adult male circumcision: effects on sexual function and sexual satisfaction in Kisumu, Kenya. J Sex Med. 2008 Nov;5(11):2610-22. DOI: 10.1111/j.1743-6109.2008.00979.x
[40] Bromage SJ, Crump A, Pearce I. Phimosis as a presenting feature of diabetes. BJU Int. 2008 Feb;101(3):338-40. DOI: 10.1111/j.1464-410X.2007.07274.x
[41] Patton JF, editor. Urology. Washington: Office of the Surgeon General and Center of Military History, United States Army; 1987. (Medical Department, United States Army. Surgery in World War II).
[42] Mallon E, Hawkins D, Dinneen M, Francics N, Fearfield L, Newson R, Bunker C. Circumcision and genital dermatoses. Arch Dermatol. 2000 Mar;136(3):350-4.
[43] O'Farrell N, Quigley M, Fox P. Association between the intact foreskin and inferior standards of male genital hygiene behaviour: a cross-sectional study. Int J STD AIDS. 2005 Aug;16(8):556-9. DOI: 10.1258/0956462054679151
[44] Wilson RA. Circumcision and venereal disease. Can Med Assoc J. 1947 Jan;56(1):54-6.
[45] Taylor PK, Rodin P. Herpes genitalis and circumcision. Brit J Ven Dis. 1975 Aug 1;51(4):274–7. DOI: 10.1136/sti.51.4.274
[46] Morris BJ, Waskett JH, Banerjee J, Wamai RG, Tobian AA, Gray RH, Bailis SA, Bailey RC, Klausner JD, Willcourt RJ, Halperin DT, Wiswell TE, Mindel A. A 'snip' in time: what is the best age to circumcise? BMC Pediatr. 2012 Feb 28;12:20. DOI: 10.1186/1471-2431-12-20
[47] Edwards SK, Bunker CB, Ziller F, van der Meijden WI. 2013 European guideline for the management of balanoposthitis. Int J STD AIDS. 2014 Aug;25(9):615-26. DOI: 10.1177/0956462414533099
[48] Schoen EJ. Circumcision as a lifetime vaccination with many benefits. J Men’s Hlth Gender. 2007 Sep;4(3):306–11. DOI: 10.1016/j.jmhg.2007.05.005
[49] Kinghorn GR, Jones BM, Chowdhury FH, Geary I. Balanoposthitis associated with Gardnerella vaginalis infection in men. Br J Vener Dis. 1982 Apr;58(2):127-9.
[50] Romero FR, Romero AW, Almeida RM, Oliveira FC Jr, Filho RT Jr. Prevalence and risk factors for penile lesions/anomalies in a cohort of Brazilian men ≥ 40 years of age. Int Braz J Urol. 2013 Jan-Feb;39(1):55-62. DOI: 10.1590/S1677-5538.IBJU.2013.01.08
[51] Verma SB, Wollina U. Looking through the cracks of diabetic candidal balanoposthitis!. Int J Gen Med. 2011;4:511-3. DOI: 10.2147/IJGM.S17875
[52] Schwartz RH, Rushton HG. Acute balanoposthitis in young boys. Pediatr Infect Dis J. 1996 Feb;15(2):176-7.
[53] Wiswell TE, Tencer HL, Welch CA, Chamberlain JL. Circumcision in children beyond the neonatal period. Pediatrics. 1993 Dec;92(6):791-3.
[54] Singh S, Bunker C. Male genital dermatoses in old age. Age Ageing. 2008 Sep;37(5):500-4. DOI: 10.1093/ageing/afn155
[55] Samuel M, Brady M, Tenant-Flowers M, Taylor C. Role of penile biopsy in the diagnosis of penile dermatoses. Int J STD AIDS. 2010 May;21(5):371-2. DOI: 10.1258/ijsa.2010.009568
[56] David N, Tang A. Efficacy and safety of penile biopsy in a GUM clinic setting. Int J STD AIDS. 2002 Aug;13(8):573-6. DOI: 10.1258/095646202760159729
[57] Toker SC, Baskan EB, Tunali S, Yilmaz M, Karadogan SK. Zoon's balanitis in a circumcised man. J Am Acad Dermatol. 2007 Aug;57(2 Suppl):S6-7. DOI: 10.1016/j.jaad.2006.02.071
[58] Kumar B, Narang T, Dass Radotra B, Gupta S. Plasma cell balanitis: clinicopathologic study of 112 cases and treatment modalities. J Cutan Med Surg. 2006 Jan-Feb;10(1):11-5. DOI: 10.1007/7140.2006.00008
[59] Moreno-Arias GA, Camps-Fresneda A, Llaberia C, Palou-Almerich J. Plasma cell balanitis treated with tacrolimus 0.1%. Br J Dermatol. 2005 Dec;153(6):1204-6. DOI: 10.1111/j.1365-2133.2005.06945.x
[60] Regauer S, Beham-Schmid C. Benign mast cell hyperplasia and atypical mast cell infiltrates in penile lichen planus in adult men. Histol Histopathol. 2014 Aug;29(8):1017-25. DOI: 10.14670/HH-29.1017
[61] Depasquale I, Park AJ, Bracka A. The treatment of balanitis xerotica obliterans. BJU Int. 2000 Sep;86(4):459-65.
[62] Singh JP, Priyadarshi V, Goel HK, Vijay MK, Pal DK, Chakraborty S, Kundu AK. Penile lichen sclerosus: An urologist's nightmare! - A single center experience. Urol Ann. 2015 Jul-Sep;7(3):303-8. DOI: 10.4103/0974-7796.150490
[63] Barbagli G, Mirri F, Gallucci M, Sansalone S, Romano G, Lazzeri M. Histological evidence of urethral involvement in male patients with genital lichen sclerosus: a preliminary report. J Urol. 2011 Jun;185(6):2171-6. DOI: 10.1016/j.juro.2011.02.060
[64] Riddell L, Edwards A, Sherrard J. Clinical features of lichen sclerosus in men attending a department of genitourinary medicine. Sex Transm Infect. 2000 Aug;76(4):311-3.
[65] Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57(1):9-30.
[66] Becker K. Lichen sclerosus in boys. Dtsch Arztebl Int. 2011 Jan;108(4):53-8. DOI: 10.3238/arztebl.2011.053
[67] Sneppen I, Thorup J. Foreskin Morbidity in Uncircumcised Males. Pediatrics. 2016 May;137(5):. DOI: 10.1542/peds.2015-4340
[68] Rickwood AM, Hemalatha V, Batcup G, Spitz L. Phimosis in boys. Br J Urol. 1980 Apr;52(2):147-50.
[69] Garat JM, Chéchile G, Algaba F, Santaularia JM. Balanitis xerotica obliterans in children. J Urol. 1986 Aug;136(2):436-7. DOI: 10.1016/S0022-5347(17)44895-6
[70] Kantere D, Löwhagen GB, Alvengren G, Månesköld A, Gillstedt M, Tunbäck P. The clinical spectrum of lichen sclerosus in male patients - a retrospective study. Acta Derm Venereol. 2014 Sep;94(5):542-6. DOI: 10.2340/00015555-1797
[71] Stewart L, McCammon K, Metro M, Virasoro R. SIU/ICUD Consultation on Urethral Strictures: Anterior urethra-lichen sclerosus. Urology. 2014 Mar;83(3 Suppl):S27-30. DOI: 10.1016/j.urology.2013.09.013
[72] Edmonds EV, Hunt S, Hawkins D, Dinneen M, Francis N, Bunker CB. Clinical parameters in male genital lichen sclerosus: a case series of 329 patients. J Eur Acad Dermatol Venereol. 2012 Jun;26(6):730-7. DOI: 10.1111/j.1468-3083.2011.04155.x
[73] Kizer WS, Prarie T, Morey AF. Balanitis xerotica obliterans: epidemiologic distribution in an equal access health care system. South Med J. 2003 Jan;96(1):9-11.
[74] Yardley IE, Cosgrove C, Lambert AW. Paediatric preputial pathology: are we circumcising enough? Ann R Coll Surg Engl. 2007 Jan;89(1):62-5. DOI: 10.1308/003588407X160828
[75] Belsante MJ, Selph JP, Peterson AC. The contemporary management of urethral strictures in men resulting from lichen sclerosus. Transl Androl Urol. 2015 Feb;4(1):22-8. DOI: 10.3978/j.issn.2223-4683.2015.01.08
[76] Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995 Mar;32(3):393-416; quiz 417-8. DOI: 10.1016/0190-9622(95)90060-8
[77] Weigand DA. Lichen sclerosus et atrophicus, multiple dysplastic keratoses, and squamous-cell carcinoma of the glans penis. J Dermatol Surg Oncol. 1980 Jan;6(1):45-50.
[78] Jayakumar S, Antao B, Bevington O, Furness P, Ninan GK. Balanitis xerotica obliterans in children and its incidence under the age of 5 years. J Pediatr Urol. 2012 Jun;8(3):272-5. DOI: 10.1016/j.jpurol.2011.05.001
[79] Kuehhas FE, Miernik A, Weibl P, Schoenthaler M, Sevcenco S, Schauer I, Tosev G, Oezsoy M, Lassmann J. Incidence of balanitis xerotica obliterans in boys younger than 10 years presenting with phimosis. Urol Int. 2013;90(4):439-42. DOI: 10.1159/000345442
[80] Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013 Feb;14(1):27-47. DOI: 10.1007/s40257-012-0006-4
[81] Becker K, Meissner V, Farwick W, Bauer R, Gaiser MR. Lichen sclerosus and atopy in boys: coincidence or correlation? Br J Dermatol. 2013 Feb;168(2):362-6. DOI: 10.1111/j.1365-2133.2012.11201.x
[82] Rickwood AM, Kenny SE, Donnell SC. Towards evidence based circumcision of English boys: survey of trends in practice. BMJ. 2000 Sep;321(7264):792-3.
[83] Griffiths D, Frank JD. Inappropriate circumcision referrals by GPs. J R Soc Med. 1992 Jun;85(6):324-5.
[84] Huntley JS, Bourne MC, Munro FD, Wilson-Storey D. Troubles with the foreskin: one hundred consecutive referrals to paediatric surgeons. J R Soc Med. 2003 Sep;96(9):449-51.
[85] Bainbridge DR, Whitaker RH, Shepheard BG. Balanitis xerotica obliterans and urinary obstruction. Br J Urol. 1971 Aug;43(4):487-91.
[86] Schinella RA, Miranda D. Posthitis xerotica obliterans in circumcision specimens. Urology. 1974 Mar;3(3):348-51. DOI: 10.1016/S0090-4295(74)80120-2
[87] Ridley CM. Genital lichen sclerosus (lichen sclerosus et atrophicus) in childhood and adolescence. J R Soc Med. 1993 Feb;86(2):69-75.
[88] Chalmers RJ, Burton PA, Bennett RF, Goring CC, Smith PJ. Lichen sclerosus et atrophicus. A common and distinctive cause of phimosis in boys. Arch Dermatol. 1984 Aug;120(8):1025-7.
[89] Bale PM, Lochhead A, Martin HC, Gollow I. Balanitis xerotica obliterans in children. Pediatr Pathol. 1987;7(5-6):617-27.
[90] Clemmensen OJ, Krogh J, Petri M. The histologic spectrum of prepuces from patients with phimosis. Am J Dermatopathol. 1988 Apr;10(2):104-8.
[91] Celis S, Reed F, Murphy F, Adams S, Gillick J, Abdelhafeez AH, Lopez PJ. Balanitis xerotica obliterans in children and adolescents: a literature review and clinical series. J Pediatr Urol. 2014 Feb;10(1):34-9. DOI: 10.1016/j.jpurol.2013.09.027
[92] Kiss A, Király L, Kutasy B, Merksz M. High incidence of balanitis xerotica obliterans in boys with phimosis: prospective 10-year study. Pediatr Dermatol. 2005 Jul-Aug;22(4):305-8. DOI: 10.1111/j.1525-1470.2005.22404.x
[93] Mattioli G, Repetto P, Carlini C, Granata C, Gambini C, Jasonni V. Lichen sclerosus et atrophicus in children with phimosis and hypospadias. Pediatr Surg Int. 2002 May;18(4):273-5. DOI: 10.1007/s003830100699
[94] Meuli M, Briner J, Hanimann B, Sacher P. Lichen sclerosus et atrophicus causing phimosis in boys: a prospective study with 5-year followup after complete circumcision. J Urol. 1994 Sep;152(3):987-9. DOI: 10.1016/S0022-5347(17)32638-1
[95] Rossi E, Pavanello P, Franchella A. Il lichen sclerosus in bambini con fimosi [Lichen sclerosus in children with phimosis]. Minerva Pediatr. 2007 Dec;59(6):761-5.
[96] Gargollo PC, Kozakewich HP, Bauer SB, Borer JG, Peters CA, Retik AB, Diamond DA. Balanitis xerotica obliterans in boys. J Urol. 2005 Oct;174(4 Pt 1):1409-12.
[97] Nyirády P, Borka K, Bánfi G, Kelemen Z. Lichen sclerosus az urológiai gyakorlatban [Lichen sclerosus in urological practice]. Orv Hetil. 2006 Nov;147(44):2125-9.
[98] Aynaud O, Piron D, Casanova JM. Incidence of preputial lichen sclerosus in adults: histologic study of circumcision specimens. J Am Acad Dermatol. 1999 Dec;41(6):923-6. DOI: 10.1016/S0190-9622(99)70247-1
[99] Nemoto K, Ishidate T. [Balanitis xerotica obliterans with phimosis in elderly patients presenting with difficulty in urination]. Hinyokika Kiyo. 2013 Jun;59(6):341-6.
[100] Micali G, Nasca MR, Innocenzi D, Schwartz RA. Penile cancer. J Am Acad Dermatol. 2006 Mar;54(3):369-91; quiz 391-4. DOI: 10.1016/j.jaad.2005.05.007
[101] Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, Carter JJ, Porter PL, Galloway DA, McDougall JK, Krieger JN. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005 Sep;116(4):606-16. DOI: 10.1002/ijc.21009
[102] Nasca MR, Lacarrubba F, Paravizzini G, Micali G. Oncogenic Human Papillomavirus Detection in Penile Lichen Sclerosus: An Update. Int STD Res Rev. 2014 Jan 10;2(1):29–37. DOI: 10.9734/ISRR/2014/7983
[103] Clouston D, Hall A, Lawrentschuk N. Penile lichen sclerosus (balanitis xerotica obliterans). BJU Int. 2011 Nov;108 Suppl 2:14-9. DOI: 10.1111/j.1464-410X.2011.10699.x
[104] Morris BJ, Gray RH, Castellsague X, Bosch FX, Halperin DT, Waskett JH, Hankins CA. The Strong Protective Effect of Circumcision against Cancer of the Penis. Adv Urol. 2011;2011:1–21. DOI: 10.1155/2011/812368
[105] Kirtschig G, Becker K, Günthert A, Jasaitiene D, Cooper S, Chi CC, Kreuter A, Rall KK, Aberer W, Riechardt S, Casabona F, Powell J, Brackenbury F, Erdmann R, Lazzeri M, Barbagli G, Wojnarowska F. Evidence-based (S3) Guideline on (anogenital) Lichen sclerosus. J Eur Acad Dermatol Venereol. 2015 Oct;29(10):e1-43. DOI: 10.1111/jdv.13136
[106] Hartley A, Ramanathan C, Siddiqui H. The surgical treatment of Balanitis Xerotica Obliterans. Indian J Plast Surg. 2011 Jan;44(1):91-7. DOI: 10.4103/0970-0358.81455
[107] Singh Thakur R, Pinjala P, Babu M. Balanitis xerotica obliterans Bxo--mimicking vitiligo. IOSR J Dent Med Sci. 2015 Aug;14(8):29-31.
[108] Wilkinson DJ, Lansdale N, Everitt LH, Marven SS, Walker J, Shawis RN, Roberts JP, Mackinnon AE, Godbole PP. Foreskin preputioplasty and intralesional triamcinolone: a valid alternative to circumcision for balanitis xerotica obliterans. J Pediatr Surg. 2012 Apr;47(4):756-9. DOI: 10.1016/j.jpedsurg.2011.10.059
[109] Homer L, Buchanan KJ, Nasr B, Losty PD, Corbett HJ. Meatal stenosis in boys following circumcision for lichen sclerosus (balanitis xerotica obliterans). J Urol. 2014 Dec;192(6):1784-8. DOI: 10.1016/j.juro.2014.06.077
[110] Wiswell TE, Geschke DW. Risks from circumcision during the first month of life compared with those for uncircumcised boys. Pediatrics. 1989 Jun;83(6):1011-5.
[111] El Bcheraoui C, Zhang X, Cooper CS, Rose CE, Kilmarx PH, Chen RT. Rates of adverse events associated with male circumcision in U.S. medical settings, 2001 to 2010. JAMA Pediatr. 2014 Jul;168(7):625-34. DOI: 10.1001/jamapediatrics.2013.5414
[112] Kacker S, Frick KD, Gaydos CA, Tobian AA. Costs and effectiveness of neonatal male circumcision. Arch Pediatr Adolesc Med. 2012 Oct;166(10):910-8. DOI: 10.1001/archpediatrics.2012.1440
[113] Morris BJ, Bailis SA, Wiswell TE. Circumcision rates in the United States: rising or falling? What effect might the new affirmative pediatric policy statement have? Mayo Clin Proc. 2014 May;89(5):677-86. DOI: 10.1016/j.mayocp.2014.01.001
[114] Schoen EJ, Colby CJ, Ray GT. Newborn circumcision decreases incidence and costs of urinary tract infections during the first year of life. Pediatrics. 2000 Apr;105(4 Pt 1):789-93. DOI: 10.1542/peds.105.4.789
[115] Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NJ; Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep. 2007 Jul 13;56(RR-6):1-54.
[116] Kelly H, Carville K, Grant K, Jacoby P, Tran T, Barr I. Estimation of influenza vaccine effectiveness from routine surveillance data. PLoS One. 2009;4(3):e5079. DOI: 10.1371/journal.pone.0005079
[117] Morris BJ. Why circumcision is a biomedical imperative for the 21(st) century. Bioessays. 2007 Nov;29(11):1147-58. DOI: 10.1002/bies.20654
[118] Ben KL, Xu JC, Lu L, Lü NQ, Cheng Y, Tao J, Liu DK, Min XD, Cao XM, Li PS. [Male circumcision is an effective "surgical vaccine" for HIV prevention and reproductive health]. Zhonghua Nan Ke Xue. 2009 May;15(5):395-402.
[119] Van Howe RS. Neonatal circumcision and penile inflammation in young boys. Clin Pediatr (Phila). 2007 May;46(4):329-33. DOI: 10.1177/0009922806295708




