Radiotherapy for Dupuytren’s Disease
1 St Lukes Cancer Centre, Royal Surrey County Hospital, Guildford, United Kingdom
Summary of recommendations
- Radiotherapy is effective at preventing contracture in the early stages of Dupuytren’s Disease, where there is no contracture (stage N) or a contracture of up to 10 degrees (N/I).
- The side-effects of radiotherapy are generally very mild, although there is a theoretical (very low) risk of radiation-induced malignancy.
- Patients with more advanced disease should not be treated with radiotherapy, and may be offered surgical release.
- Due to the variable progression of this disease, only patients whose disease has progressed within the last 6–12 months should be treated.
Radiotherapy for benign diseases
Radiotherapy is the medical use of ionising radiation (generally X-rays or electrons). It is mostly used to treat cancer, but due to its anti-inflammatory and anti-proliferative effects it is also used to treat benign disease, including benign tumours. The doses used in benign conditions are generally much lower than those used for cancer, and the side-effects are correspondingly much less severe.
The use of radiotherapy for benign conditions has declined in recent decades. This may be largely due to the increased availability of alternative medical therapies, advances in surgery, and an increased awareness of the theoretical increased risk of radiation-induced malignancy.
The use of radiotherapy for benign conditions is particularly prevalent in Germany. A patterns of care study from 134 German institutions, surveyed in 1994–96, showed that approximately 20,000 patients were treated for benign conditions annually, with 146 of these being for Dupuytren’s Disease [1].
The use of RT for benign conditions is currently being evaluated by a UK Royal College of Radiologists working group, with a report from that group expected by mid-2014.
Radiotherapy notes:
a. Dose is defined as energy in Joules absorbed by 1 kilogram of tissue, and is expressed as Gray (Gy). The total dose for the complete course is divided into daily treatments (fractions). So, for example, five daily doses of 4 Gy per dose would be expressed as “20 Gy in 5 fractions over 1 week”.
b. Penetration: The ability of the radiation to penetrate the tissue is expressed as the “energy” of the beam.
c. Type of radiation: For Dupuytren’s Disease either X-rays (at a fairly low energy) or high-energy electrons are used, and the choice depends on the availability of equipment, workflow, cost, and the doses required at particular depths in the tissue.
Effectiveness
There are numerous retrospective studies in the literature going back many decades that have indicated the efficacy of radiotherapy for Dupuytren’s Disease [2], [3], [4], [5], [6], [7]. However, their usefulness is limited by baseline differences in patient and disease characteristics, radiotherapy doses and fractionations, definitions of endpoints, and short follow-up periods. The staging of Dupuytren’s Disease is illustrated in table 1, where stage N is disease with no contracture, stage N/I is disease with up to 5–10 degrees of contracture, and subsequent stages indicate disease with more severe contracture.
| Stage | Clinical symptoms | Extent of extensions deficit |
|---|---|---|
| N | Nodules, cords, skin retraction etc. | None |
| N/I* | As stage N + deformity of fingers | 1–10° |
| I | As stage N + deformity of fingers | 11–45° |
| II | As stage N + deformity of fingers | 46–90° |
| III | As stage N + deformity of fingers | 91–135° |
| IV | As stage N + deformity of fingers | >135° |
| *In some papers, N/I is defined as 1–5˚ of extension deficit. | ||
A retrospective study with a median follow-up of 6 years looked at 96 patients (142 hands) [9]. 70% had stage N or N/I disease. The patients were treated to a total dose of 30 Gy in 10 fractions, which was split into two phases of 15 Gy in 5 fractions over 1 week, with a six week gap between the phases. At the latest follow-up, 11% of hands overall showed stage progression. Of those with at least 5 years of follow-up, 23% experienced any form of progression, including increasing symptoms or growing nodules. Only minor side-effects were noted.
A retrospective study with a median follow-up of 10 years looked at 99 patients (176 hands) treated with the same dose and fractionation (30 Gy in 10 fractions), and demonstrated progressive disease in 16% of patients with stage N, 33% in stage N/I, 65% in stage I, and 83% in stage II [10].
A third retrospective study [11], with a median follow-up of 13 years looked at the outcomes of 135 patients (208 hands) treated with 30 Gy in 10 fractions, and demonstrated progressive disease in 31% overall, with progression by stage of: N = 13%, N/I = 30%, I = 62%, II = 86%, III/IV = 100%. Additionally, it was noted that the outcome was significantly better if the disease was treated within one year of the appearance of symptoms as compared with waiting for more than two years.
A prospective trial randomising patients between two dose levels (with no control group) looked at 129 patients (198 hands) [12]. All of them had disease that had progressed within the last six months. Patients were randomised to a total of 30Gy in 10 fractions (as above), or to 21 Gy in 7 fractions (given on alternate days over a period of 15 days). The treatment was generally well tolerated, with acute grade 1 toxicity of 38% and grade 2 toxicity of 6%. There was a chronic toxicity rate of 5% at 12 months. At 12 months follow-up, the overall treatment failure rate was 8%, with 2% needing corrective surgery. Progression by stage was: 0% in stage N, 3% in N/1, 15% in St 1, 40% in St II. There was no significant difference in efficacy or toxicity between the two dose groups.
A long-term follow-up of this study, published as a textbook chapter [13], looked at the outcomes of patients followed up for at least 5 years (median follow-up of 102 months). 406 patients (812 hands) were treated with radiotherapy, (total dose 21 Gy or 30 Gy, as above), and a non-randomised control group of 83 patients (166 hands), consisting of patients who chose to be observed rather than treated. All had progressive disease in the last 6–12 months. Side-effects in the irradiated group were: Acute toxicity in 28% (2% grade 2); chronic toxicity in 14% (all grade 1). Acute and chronic toxicity rates were increased in the 21 Gy group compared with the 30 Gy group. Overall disease progression by stage was: stage N = 10%, N/I = 41%, I = 58%, II-IV = 89%. Regarding efficacy, a significant reduction in disease progression and the need for surgery was demonstrated in both treatment groups compared with the control group, although there was no significant difference between the two treatment groups (Table 2).
| Dose | Regression or stabel disease (%) |
Progression (all clinical signs, %) |
Surgery (%) |
|---|---|---|---|
| Control (n=122) | 38 | 62 | 30 |
| 21 Gy (n=293) | 76 | 24 | 12 |
| 30 Gy (n=245) | 80 | 19.5 | 8 |
The author’s radiotherapy process
1. Eligibility (ALL of)
- Nodules and/or cords
- No evidence of contracture (stage N), or contracture <10 degrees (stage N/I)
- Progressive disease in the last 6–12 months
2. History
To include causal factors, family history, and other associated conditions e.g. plantar fibromatosis, Peyronie’s disease.
3. Examination
- Hands – Nodules, cords, skin retraction, fixed extension deficits
- Feet – evidence of plantar fibromatosis
- Other – as indicated by the history
4. Informed consent
a. Skin changes – redness, soreness, dryness, subcutaneous fibrosis – generally mild, risk of chronic skin changes
b. Risk of malignancy:
- The radiation dose is low and given to a peripheral area of the body
- There have been no documented cases of malignancy as a result of radiotherapy for Dupuytren’s Disease. It is certainly low risk, perhaps average 0.1% lifetime risk [14].
- The risk is age-associated, so should be more cautious in younger patients. Probably minimal risk over the age of 60 years
5. Treatment
This can be delivered with either low-energy X-rays or with electrons. There is no evidence of a difference in effectiveness between these two treatments.
a. Planning
All palpable nodules and cords are outlined on the skin and an appropriate margin (e.g. 1–2 cm) is added in order to encompass subclinical disease. An appropriate X-ray or electron energy (and or tissue-equivalent bolus) is selected, depending on the estimated depth of disease. Lead shielding is used in order to protect those parts of the hand that are not involved with disease.
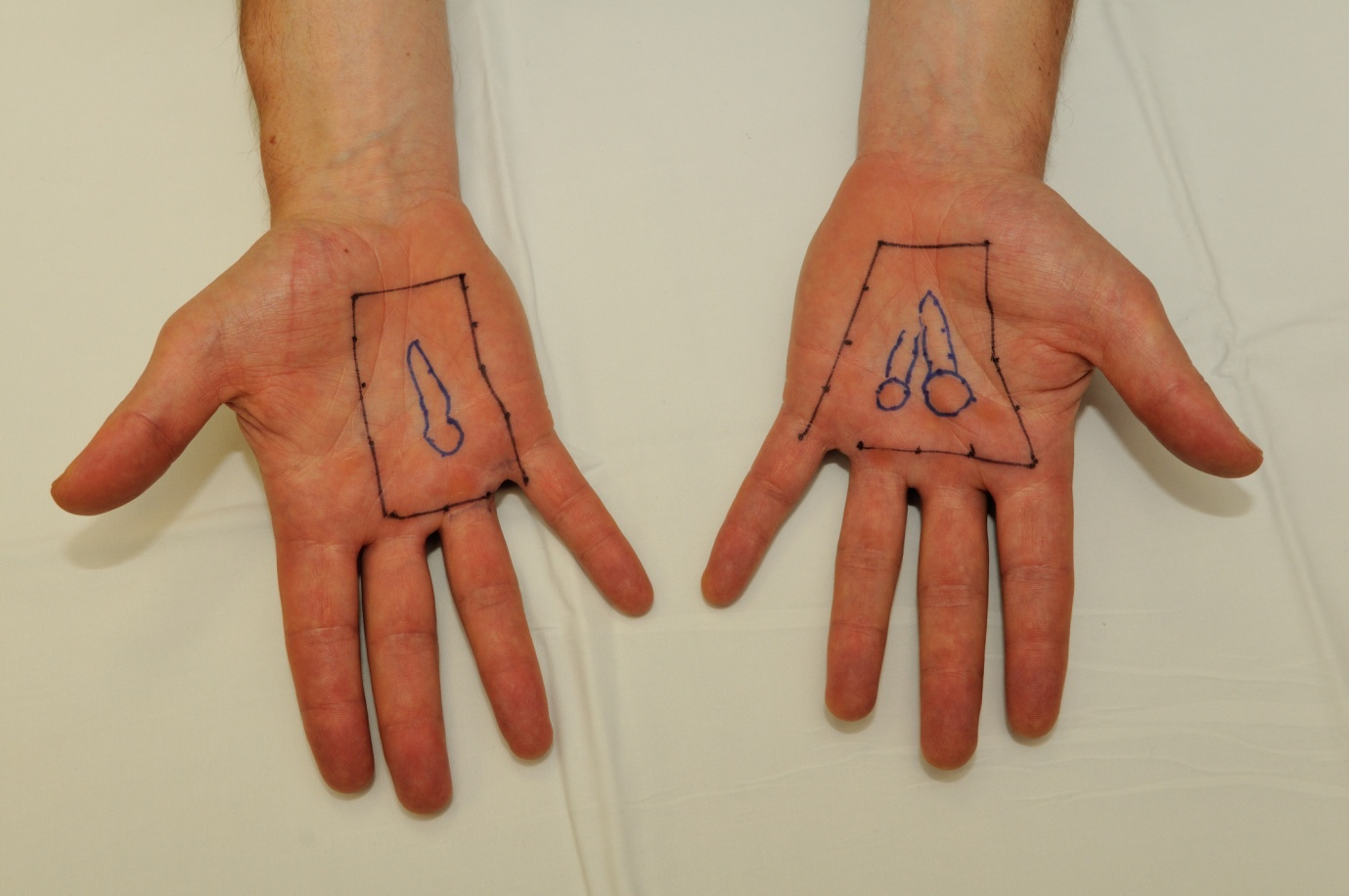
b. Treatment
i. Positioning
The patients can be positioned either sitting, standing or lieing on a couch. The machine is moved close the hand and lead shielding is put in place to protect the areas of the palm that do not require irradiating. With electron treatment, the shielding is within the machine.

ii. Dose
The total dose used is 30 Gy in 10 fractions. This is divided into two phases:
- Phase 1 = 15 Gy in 5 fractions given over 1 week.
- There is then a gap of 6–12 weeks.
- Phase 2 = 15 Gy in 5 fractions given over 1 week.
iii. Timing
The treatment takes about 10–20 minutes each day, and is given on consecutive weekdays (monday to friday) for five days. The patient does not feel any pain during the treatment.
c. Side-effects
The patient is counselled that the treatment does not make him/her feel drowsy or dizzy, so if they drive to the treatment centre then they can drive home. There may be minor skin reddening, soreness, and dryness, and they are told that they can rub simple moisturisers e.g. E45 or aqueous cream on the area. They are also told that they can continue with all normal activities during and in the post-treatment phase. Occasionally there may be a patient who performs very traumatic work with his or her hands (e.g. building work) that should use extra protection (i.e. gloves) around that time.
d. Follow-up
Patients are offered a follow-up appointment at 3 months, although long-term follow-up is achieved by a follow-up questionnaire for the purposes of assessing long-term outcomes from this treatment.
References
[1] Seegenschmiedt MH, Katalinic A, Makoski H, Haase W, Gademann G, Hassenstein E. Radiation therapy for benign diseases: patterns of care study in Germany. Int J Radiat Oncol Biol Phys. 2000 Apr 1;47(1):195-202.[2] Finney R. Dupuytren’s Contracture. Br J Radiol. 1955;28(335):610-6. DOI: 10.1259/0007-1285-28-335-610
[3] Wasserburger K. Zur Therapie der Dupuytrenschen Kontraktur. Strahlenther. 1956;100:546-60.
[4] Lukacs S, Braun-Falco O, Goldschmidt H. Radiotherapy of benign dermatoses: indications, practice, and results. J Dermatol Surg Oncol. 1978 Aug;4(8):620-5. DOI: 10.1111/j.1524-4725.1978.tb00512.x
[5] Hesselkamp J, Schulmeyer M, Wiskemann A. Rontegntherapie der Dupuytrenschen Kontraktur im Stadium I. Therapiewoche. 1981;31:6337-8.
[6] Kohler AH. Die Strahlentherapie der Dupuytrenschen Kontraktur. Radiobiol Radiother. 1984;25:851-3.
[7] Herbst M, Regler G. Dupuytrensche Kontraktur. Radiotherapie der Fruhstadien. Strahlentherapie. 1986;161:143-7.
[8] Tubiana R, Michon J, Thomine JM. Evaluation chiffree des deformations dans la maladie de Dupuytren. In: Maladie du Dupuytren. Paris:G.E.G. Expansion Scientificque Francaise; 1966.
[9] Keilholz L, Seegenschmiedt MH, Sauer R. Radiotherapy for prevention of disease progression in early-stage Dupuytren's contracture: initial and long-term results. Int J Radiat Oncol Biol Phys. 1996 Nov 1;36(4):891-7. DOI: 10.1016/S0360-3016(96)00421-X
[10] Adamietz B, Keilholz L, Grünert J, Sauer R. [Radiotherapy of early stage Dupuytren disease. Long-term results after a median follow-up period of 10 years]. Strahlenther Onkol. 2001 Nov;177(11):604-10.
[11] Betz N, Ott OJ, Adamietz B, Sauer R, Fietkau R, Keilholz L. Radiotherapy in early-stage Dupuytren's contracture. Long-term results after 13 years. Strahlenther Onkol. 2010 Feb;186(2):82-90. DOI: 10.1007/s00066-010-2063-z
[12] Seegenschmiedt MH, Olschewski T, Guntrum F. Radiotherapy optimization in early-stage Dupuytren's contracture: first results of a randomized clinical study. Int J Radiat Oncol Biol Phys. 2001 Mar 1;49(3):785-98.
[13] Seegenschmiedt MH, Keilholz L, Wielpütz M, Schubert C, Fehlauer F. Long-term outcome of radiotherapy for early stage Dupuytren’s disease: A phase III clinical study. In: Eaton C, Seegenschmiedt MH, Bayat A, Gabbiani G, Werker P, Wach W, editors. Dupuytren’s disease and related hyperproliferative disorders. Berlin Heidelberg: Springer; 2012. p. 349-371.
[14] Jansen JTM, Broerse JJ, Zoetelief J, Klein C, Seegenschmiedt HM. Estimation of the carcinogenic risk of radiotherapy of benign diseases from shoulder to heel. Radiother Oncol. 2005;76(3):270–7. DOI: 10.1016/j.radonc.2005.06.034



