Basics in clinical neurophysiology: Nerve conduction studies and needle electromyography in nerve entrapment syndromes
Christian Bischoff 2
1 Department of Neurology, Innsbruck Medical University, Innsbruck, Austria
2 Neurologische Gemeinschaftspraxis am Marienplatz, München, Germany
Abstract
The following chapter provides an overview about the principles of nerve conduction studies and needle electromyography. Emphasis is given to its application in the diagnostic evaluation of entrapment syndromes of the upper extremity. Typical findings in frequent entrapment syndromes are described as well as grading systems. Relevant indications and limitations are discussed.
Introduction
Neurophysiological studies form a relevant and well-established part in the diagnosis and work-up of nerve entrapment syndromes. They extend the clinical examination and help to answer several questions (mod. from [1]):
- Does a nerve lesion at an entrapment site exist?
- Where precisely is the lesion located?
- Are other nerves involved, which may explain patient’s signs and symptoms?
- What type of lesion is it – axonal or a demyelinating?
- Is there a generalized lesion of peripheral nerves, e.g. polyneuropathy?
- Is the lesion acute, subacute or chronic?
- What is the severity of the lesion?
Additional questions, which can only partially be answered, are:
- What is the prognosis of an untreated entrapment syndrome?
- What treatment modality is necessary – surgery or conservative treatment?
- Was the treatment effective?
To answer the questions above, nerve conduction studies (NCS) and needle electromyography (EMG) are necessary.
General principles of nerve conduction studies
Motor and sensory nerve conduction studies (NCS) are performed [2], [3]. The type of study depends on the nerve studied (motor, sensory or mixed nerve) and on the site of recording, e.g. the motor portion of a mixed nerve is studied when signals are recorded form a muscle, the sensory part when signals are recorded of pure sensory nerve branches. The signals are recorded using a differential amplifier, which means that the difference between electric potentials, picked up by two electrodes, is recorded. Filtering and amplification of signals is necessary to obtain optimal results.
In NCS the nerve is electrically stimulated by a bipolar surface stimulator. The stimulation occurs underneath the cathode. The stimulation depends on the duration and intensity of the electrical pulse and must be supramaximal. Duration of the stimulus typically is 0.1 ms in sensory and 0.2 ms in motor NCS. Most stimulators keep the stimulating current constant. Maximum stimulator output is 100 mA. The more superficial nerves the easier they are stimulated. As a general rule, only myelinated nerve fibres can be investigated.
Factors affecting nerve conduction studies and reference values
NCSare strongly influenced by temperature. Skin temperature should be measured and the extremity be warmed if the temperature is <32°C. Gender has no clinically relevant influence on NCS, but nerve conduction gets slightly slower at ages above 75 ys.
Each laboratory usually has its own reference values for standard NCS. Reference values form our laboratory are listed in table 1.
| Nerve | Q | DML (ms) |
NCV (m/s) |
Amp (mV-µV) |
F-latency (ms) |
|---|---|---|---|---|---|
| N. medianus | m | ≤4,2 | ≥49 | ≥5 | ≤31 |
| N. ulnaris - forearm | m | ≤3,3 | ≥50 | ≥4 | ≤30 |
| N. radialis - elbow | m | ≥43 | ≥4 | ||
| N. medianus | s | ≥45 | ≥10 | ||
| N. ulnaris | s | ≥45 | ≥8 | ||
| N. radialis | s | ≥55 | ≥16 | ||
| Legend: Q = quality; m = motor; s = sensory; DML = distal motor latency; NCV = nerve conduction velocity; Amp = amplitude (motor in mV, sensory in µV); F = F-wave study | |||||
Motor nerve conduction studies (mNCS)
In mNCS compound motor action potentials (CMAP) are recorded from a muscle innervated by the nerve under investigation. Surface electrodes are used and the “active” (recording) electrode is placed over the muscle belly, the other (reference) electrode over the tendon at the insertion of the muscle (belly-tendon electrode placement). Figure 1 shows the standard electrode placement to study the CMAP of the abductor pollicis brevis muscle and stimulation of the median nerve at the wrist.
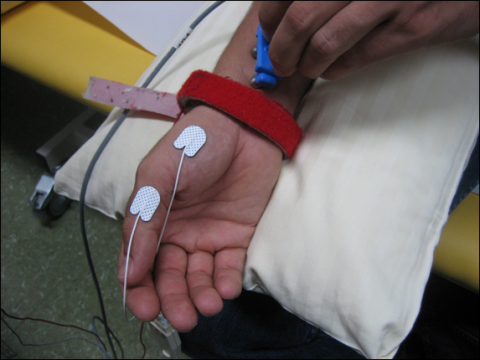
First, the nerve is stimulated at distal site. A predefined distance between stimulating and active recording electrode is required. In median and ulnar mNCSthe distal stimulation is performed at the wrist. In a second step, the nerve is stimulated at one or more proximal sites. Stimulation intensity has to be supramaximal, i.e. the CMAP amplitude should be as high as possible. Several parameters are evaluated: distal motor latency (DML), nerve conduction velocity (NCV) and amplitudes of the CMAPafter distal and proximal stimulation, as well as duration and shape of the CMAP. Figure 2 shows the result of a normal median nerve mNCS.
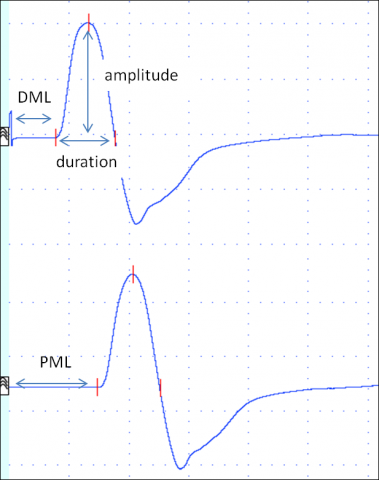
DML is the time in milliseconds from distal stimulation to the onset of the CMAP. It reflects the conduction along the distal nerve segment, the time of neuromuscular transmission and the conduction along muscle fibres.
NCV is calculated by dividing the distance between distal and proximal stimulation sites by the latency difference between proximal and distal stimulation. It is expressed in meters/second. It reflects the nerve conduction of the fastest nerve fibers of the segment investigated.
Amplitude of the CMAP elicited by supramaximal stimulation should be similar following distal and proximal stimulation in mNCS. The amplitude reflects the number of nerve fibres stimulated and the number of muscle fibres activated. Shape and duration should also be similar after distal and proximal stimulation.
Duration and shape of the CMAP reflect the range of conduction velocities of the nerve fibres of the nerve studied.
Interpretation of motor nerve conduction studies in entrapment syndromes
In the clinical setting of nerve entrapment syndromes mNCS assess the function of the myelin sheath and of the axons. Prolonged DMLs or reduced NCVs usually indicate de- and remyelination, while reduced CMAP amplitudes at distal and proximal stimulation sites suggest axonal damage. Axonal damage, however, needs to be confirmed be EMG. A conduction block is defined as significant reduction of the CMAP (usually >50%) following proximal stimulation compared to the CMAP amplitude following distal stimulation. It indicates focal demyelination, which is a frequent finding in acute nerve compression.
Sensory nerve conduction studies (sNCS)
Orthodromic or antidromic nerve conduction studies are performed to study sensory nerves. In antidromic studies amplitudes are usually higher thanin orthodromic studies, otherwise both methods are comparable. In antidromic studies of e.g. the median or ulnar nerves, the sensory nerve action potential (SNAP) may be recorded using ring electrodes placed around a finger, and the nerves are stimulated at the wrist. In orthodromic studies, surface electrodes are placed over the nerve at the wrist, and finger nerves are stimulated by ring electrodes.
Figure 3 shows the standard electrode placement in antidromic and orthodromic median nerve sNCS.
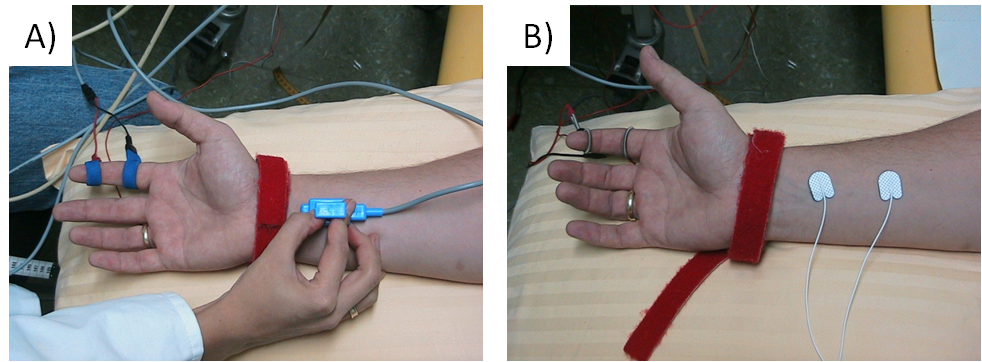
Several parameters are evaluated: onset (or peak) latency, sNCV and amplitude (Figure 4).
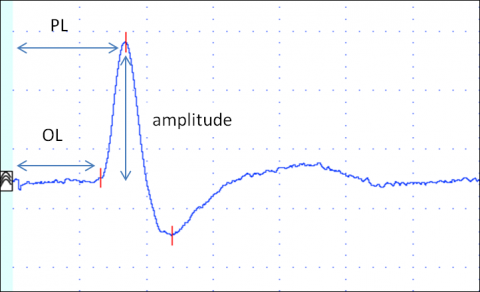
Onset latency (ms) is the time in milliseconds from stimulation to the onset of the SNAP. Onset latency reflects the conduction time of the fastest conducting nerve fibres.
Peak latency (ms) is the time in milliseconds from stimulation to the negative peak of the SNAP (negative is upwards in NCS). It reflects the conduction along the majority of the axons.
sNCV (in meters/second) is calculated by dividing the distance between stimulating and recording electrodes by onset (or peak) latency. It reflects the fastest nerve conduction velocity.
Amplitude (in µV) reflects the number of conducting axons.
Interpretation of sensory nerve conduction studies in entrapment syndromes
In the clinical setting of nerve entrapment syndromes sensory nerve conduction studies assess the function of the myelin sheath and of the axons. Prolonged latencies or reduced NCVs usually indicate de- and remyelination, while reduced amplitudes suggest axonal damage. However, demyelination can also result in reduced SNAPsin sensory studies.
General principles of needle electromyography (EMG)
Electric potentials produced by muscle fibres can also be recorded using intramuscular needle electrodes [2], [4], [5]. Pathologic EMG findings are only seen in axonal nerve lesions.
Concentric or monopolar needle electrodes are inserted into the muscle under investigation and the electric activity recorded is displayed on a monitor and also transformed into acoustic signals. In patients with anticoagulation EMG should only be performed with caution.
Typically, EMG is recorded when the muscle is at rest, during a weak contraction and during a strong contraction. With the muscle at rest, spontaneous activity is assessed. In a healthy muscle there is only a brief burst of EMG signal when the needle is moved within the muscle. This is called insertional activity. Following an axonal injury, several forms of pathological spontaneous activity may be found. Fibrillation potentials (fibs) and positive sharp waves (psw) are seen 14–21 days following an axonal nerve lesion. They usually persist for 6–12 month, although some spontaneous activity may persist for many years. Complex repetitive discharges and myokymic discharges are sometimes seen in chronic lesions. However, all types of spontaneous activity can occur in other disorders of nerve or muscle as well.
The morphology of motor unit action potentials (MUAPs) is analysed during a weak voluntary contraction (MUAP analysis). Several MUAPs should be recorded within a muscle and amplitude, duration, number of phases and turns are the most important parameters analysed. In f axonal nerve lesions, the following MUAP changes are typically seen (Figure 5A–E): low-amplitude polyphasic potentials indicate early re-innervation following a (sub)total nerve lesion, polyphasic normal or high amplitude MUAPs are seen in subacute neurogenic lesions after partial nerve damage and during later stages of re-innervation. Satellite potentials are the electric footprint of collateral sprouting. MUAPs of high amplitude and long duration indicate a chronic neurogenic lesion without ongoing re-innervation.
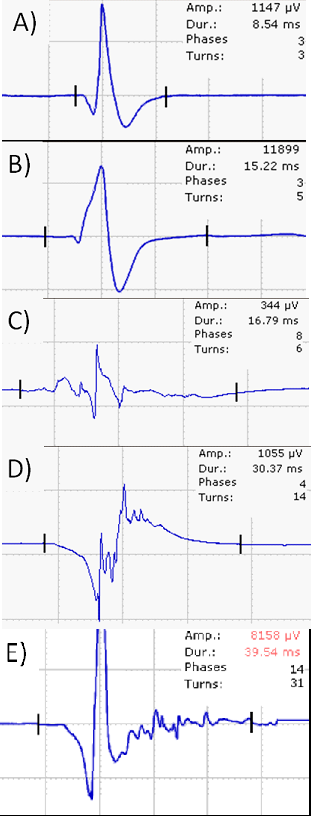
In the next step motor unit recruitment and interference pattern can be analysed. Reduced recruitment is seen in nerve lesions. To differentiate this from central weakness the firing frequency of motor units (MU) is used. In peripheral nerve lesions the firing frequency is higher (>20/s) than in central lesions (about 10/s). A normal muscle has a full interference pattern; a less dense interference pattern indicates loss of MU. A less dense, high amplitude interference patter is seen in chronic nerve lesions.
Nerve conduction studies and EMG in entrapment syndromes
The initial lesion in entrapment syndromes typically is a focal damage to the myelin sheaths. Therefore, NCS are the mainstay in the diagnosis of all entrapment syndromes that are accessible to NCS. The typical finding is conduction block in an acute lesion or focal slowing of nerve conduction at the site of entrapment. Needle EMG is necessary in entrapment syndromes, which are not reliably accessible to NCS or in cases when axonal damage is suspected. Depending on the clinical setting, non-affected nerves can be additionally studied to exclude generalized disorders such as polyneuropathies.
Guidelines and standards for nerve conduction studies and validated neurophysiological grading scales exist only for carpal tunnel syndrome and ulnar neuropathy at the elbow [6], [7].
Typical findings in CTS
Median nerve sNCV is slowed, either absolute or in comparison to the ipsilateral ulnar (or radial) nerve. DML of the median nerve is prolonged and mNCV in the forearm is normal. CMAP from the abductor pollicis brevis muscle is either normal or in severe cases reduced or absent. The ulnar nerve motor and sensory NCS are normal. Sensitivity and specificity of NCSin CTS are high. Aneurophysiologcial classification has been proposed by Padua et al. [8], [9] (Table 2) and shows a good correlation with clinical severity [10].
| Grade | Neurophysiological findings |
|---|---|
| Negative | normal findings on all tests |
| Minimal | abnormal segmental or comparative tests only |
| Mild | abnormal digit/ wrist sensory nerve conduction velocity and normal distal motor latency |
| Moderate | abnormal digit/ wrist sensory nerve conduction velocity and abnormal distal motor latency |
| Severe | absence of sensory response and abnormal distal motor latency |
| Extreme | absence of motor and sensory response |
Typical findings in ulnar neuropathy at the elbow
The mNCV of the ulnar nerve around the elbow is slowed, either absolutely or in comparison to the NCV in the forearm. Conduction block or a change in CMAP shape can be seen following stimulation of the nerve above the elbow. Distal SNAP can be normal or reduced/absent in case of axonal damage. CMAP amplitude from the abductor digitiminimi or first dorsal interosseus muscles is either normal or in severe cases reduced or absent. When only temporary symptoms or permanent sensory signs without weakness are present, the sensitivity is low. A neurophysiologcial classification has been proposed by Padua et al. [11] (Table 3).
| Grade | Neurophysiological findings |
|---|---|
| Negative | normal findings on all tests; |
| Mild | slowing of ulnar motor nerve conduction velocity across elbow and normal ulnar sensory nerve action potential |
| Moderate | slowing of ulnar motor nerve velocity across elbow and reduced amplitude of ulnar sensory nerve action potential |
| Severe | slowing of ulnar motor nerve conduction velocity across elbow and absence of ulnar sensory nerve action potential |
| Extreme | absence of hypothenar motor (and sensory) response |
Typical findings in ulnar neuropathy at the wrist (Loge de Guyon)
The findings depend on the location of the nerve damage [12], [13]. The DML to the abductor digitiminimi and/or first dorsal interosseus muscle is prolonged (side-to-side comparison). Conduction block can be found when the nerve is stimulated distal and proximal of the wrist. Distal ulnar nerve sNCV can be normal or slowed, the amplitude reduced or the SNAP can be absent in very proximal compression of the nerve. EMG localises the lesion in severe cases.
Findings in other entrapment syndromes of the upper extremity
The NCV of the radial nerve in the forearm can be slowed in posteriorinterosseus nerve compression syndrome or conduction block can be found [14]. In Kiloh-Nevinesyndrome, the interosseus anterior nerve DML to the pronator quadrates muscle can be prolonged. However, EMG is usually necessary to ascertain the clinical suspicion. In other, more proximal entrapment syndromes, EMG is the method of choice. NCS can assist to differentiate entrapment syndromes from generalized disorders.
When should nerve conduction be performed
NCS are essential to diagnose and localize nerve compressions. However, NCS should not be used to predict treatment modality [15] because as it has been shown that spontaneous recovery is possible and independent of neurophysiological severity [16], [17].
NCS are also used to assess the efficacy of treatment. As a rule, myelin damage recovers quicker than axonal damage. Follow-up investigations should be performed at the earliest after 3 month. Earlier studies are likely to be non-informative. Some improvement of nerve conduction (DML, NCV, amplitude) can be seen after successful treatment but NCS may remain unchanged despite clinical improvement [18]. However, follow-up studies are relevant in patients who do not recover.
References
[1] Seror P. Sonography and electrodiagnosis in carpal tunnel syndrome diagnosis, an analysis of the literature. Eur J Radiol. 2008 Jul;67(1):146-52. DOI: 10.1016/j.ejrad.2007.06.017[2] Kane NM, Oware A. Nerve conduction and electromyography studies. J Neurol. 2012 Jul;259(7):1502-8. DOI: 10.1007/s00415-012-6497-3
[3] Whittaker RG. SNAPs, CMAPs and F-waves: nerve conduction studies for the uninitiated. Pract Neurol. 2012 Apr;12(2):108-15. DOI: 10.1136/practneurol-2011-000126
[4] Whittaker RG. The fundamentals of electromyography. Pract Neurol. 2012 Jun;12(3):187-94. DOI: 10.1136/practneurol-2011-000198
[5] Daube JR, Rubin DI. Needle electromyography. Muscle Nerve. 2009 Feb;39(2):244-70. DOI: 10.1002/mus.21180
[6] Jablecki CK, Andary MT, Floeter MK, Miller RG, Quartly CA, Vennix MJ, Wilson JR; American Association of Electrodiagnostic Medicine; American Academy of Neurology; American Academy of Physical Medicine and Rehabilitation. Practice parameter: Electrodiagnostic studies in carpal tunnel syndrome. Report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2002 Jun 11;58(11):1589-92. DOI: 10.1212/WNL.58.11.1589
[7] American Association of Electrodiagnostic Medicine; American Academy of Neurology; American Academy of Physical Medicine and Rehabilitation. Practice parameter: electrodiagnostic studies in ulnar neuropathy at the elbow. American Association of Electrodiagnostic Medicine, American Academy of Neurology, and American Academy of Physical Medicine and Rehabilitation. Neurology. 1999 Mar 10;52(4):688-90. DOI: 10.1212/WNL.52.4.688
[8] Padua L, Padua R, Aprile, Tonali P. Italian multicentre study of carpal tunnel syndrome. Differences in the clinical and neurophysiological features between male and female patients. J Hand Surg Br. 1999 Oct;24(5):579-82. DOI: 10.1054/JHSB.1999.0255
[9] Padua L, LoMonaco M, Gregori B, Valente EM, Padua R, Tonali P. Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol Scand. 1997 Oct;96(4):211-7. DOI: 10.1111/j.1600-0404.1997.tb00271.x
[10] Giannini F, Cioni R, Mondelli M, Padua R, Gregori B, D'Amico P, Padua L. A new clinical scale of carpal tunnel syndrome: validation of the measurement and clinical-neurophysiological assessment. Clin Neurophysiol. 2002 Jan;113(1):71-7. DOI: 10.1016/S1388-2457(01)00704-0
[11] Padua L, Aprile I, Mazza O, Padua R, Pietracci E, Caliandro P, Pauri F, D'Amico P, Tonali P. Neurophysiological classification of ulnar entrapment across the elbow. Neurol Sci. 2001 Feb;22(1):11-6. DOI: 10.1007/s100720170030
[12] Cowdery SR, Preston DC, Herrmann DN, Logigian EL. Electrodiagnosis of ulnar neuropathy at the wrist: conduction block versus traditional tests. Neurology. 2002 Aug;59(3):420-7. DOI: 10.1212/WNL.59.3.420
[13] Kim DH, Kang YK, Hwang M, Kwon HK, Lee HJ, Kim BG. Reference values of fractionated neurography of the ulnar nerve at the wrist in healthy subjects. Clin Neurophysiol. 2005 Dec;116(12):2853-7. DOI: 10.1016/j.clinph.2005.08.002
[14] Werner P, Furtner M, Löscher WN, Gotwald T, Piza-Katzer H. A case of posterior interosseous nerve palsy: good recovery despite diagnostic delay. J Neurol Neurosurg Psychiatry. 2007 Dec;78(12):1408-9. DOI: 10.1136/jnnp.2007.126896
[15] Naranjo A, Ojeda S, Araña V, Baeta P, Fernández-Palacios J, García-Duque O, Rodríguez-Lozano C, Carmona L. Usefulness of clinical findings, nerve conduction studies and ultrasonography to predict response to surgical release in idiopathic carpal tunnel syndrome. Clin Exp Rheumatol. 2009 Sep-Oct;27(5):786-93.
[16] Padua L, Aprile I, Caliandro P, Foschini M, Mazza S, Tonali P. Natural history of ulnar entrapment at elbow. Clin Neurophysiol. 2002 Dec;113(12):1980-4. DOI: 10.1016/S1388-2457(02)00295-X
[17] Padua L, Padua R, Aprile I, Pasqualetti P, Tonali P; Italian CTS Study Group. Carpal tunnel syndrome. Multiperspective follow-up of untreated carpal tunnel syndrome: a multicenter study. Neurology. 2001 Jun;56(11):1459-66. DOI: 10.1212/WNL.56.11.1459
[18] Schrijver HM, Gerritsen AA, Strijers RL, Uitdehaag BM, Scholten RJ, de Vet HC, Bouter LM. Correlating nerve conduction studies and clinical outcome measures on carpal tunnel syndrome: lessons from a randomized controlled trial. J Clin Neurophysiol. 2005 Jun;22(3):216-21. Available from: https://research.vu.nl/files/2109616/187007.pdf



