Operating on nerves: Surgical approaches, primary and secondary repair, nerve grafting and nerve transfer
1 Orthopaedic Surgery, Queen Elizabeth University Hospital, Glasgow, Vereinigtes Königreich
Abstract
Exploration and repair of nerves requires specialized equipment, detailed anatomical knowledge, and expertise in microsurgery. This chapter includes details of a primary and secondary repair of nerves. Nerve repairs are most often carried out using sutures, but alternative methods of coaptation are reviewed. The management of nerve defects is discussed and techniques for nerve grafting are described.
Operating on nerves
Anaesthesia
Nerve exploration and repair procedures are often lengthy with extensive exposures being required. Isolated digital nerve repairs may be possible under local anaesthetic ring block. Some nerve repairs in the hand or forearm can be performed under brachial plexus block, but most cases require a general anaesthetic. If nerve grafts are needed from other limbs then general anaesthetic is essential. Neuromuscular blockers have to be avoided if intra-operative stimulation of nerves is to be used.
Instruments
Magnification with loupes (approximately 3 times) is necessary for most operations on nerves. For repair of smaller nerves an operating microscope is advantageous to aid optimal alignment. It has not, however, been conclusively shown that repair using a microscope leads to improved outcome. Fine instruments, including jewelers’ forceps, microsurgical needle holders and scissors are needed. If a nerve end needs trimming, then a scalpel with a large blade may be used or a Meyer nerve holding and trimming instrument will provide a particularly clean cut (Figure 1).
used to trim the end of a disrupted nerve before repair with a nerve graft
The nerve is grasped with the appropriate diameter of ring and then the blade is passed through the slot. It can only be used if the nerve is to be completely divided.
Surgical approaches to nerves innervating the hand
Extensive exposures are often needed for exploration of nerves. Nerves are longitudinal structures in limbs. Adequate exposure both above and below the damaged area is required together with adjacent structures. In cases where exploration is carried out more than 2 weeks after injury then there will be scarring. It is important to start dissection in unscarred tissue, proximally and distally, identify the nerve and then trace it into the area of injury. Dissection is easier in a bloodless field if it is possible to use a tourniquet. Exposure of individual nerves is described below (also see chapter: Nerves: Structure, anatomy and response to injury).
Median nerve
In the upper arm the median nerve can be easily exposed through a medial longitudinal incision. The nerve is found lying anterior to the brachial artery proximally and medial to the artery lower down. For exposure in the antecubital fossa the incision is extended distally in an S-shape. The nerve can be traced distally between the head of pronator teres (see chapter: Median nerve compression: Pronator and anterior interosseous syndromes).
Full exposure in the proximal third of the forearm is difficult and requires significant division of overlying muscle. In the mid forearm the median nerve is most easily exposed through an incision on the anteromedial aspect. The plane between the flexor carpi ulnaris (FCU) and the flexor digitorum superficialis (FDS) is developed down to the ulnar nerve and artery. Working laterally underneath FDS, the nerve is found lying between FDS and FDP. The distal part of the anterior interosseous nerve can also be exposed via this approach.
At the wrist the median nerve lies fairly superficial under the tendons of flexor carpi radialis (FCR) and palmaris longus (PL). It can be traced distally by opening the carpal canal until its branches in the palm of the hand.
Ulnar nerve
In the upper arm the ulnar nerve can be exposed through a longitudinal medial incision and is found to be lying on the medial border of the triceps muscle. At the elbow it is superficial behind the medial epicondyle and can be traced distally for a few centimetres by splitting the heads of FCU. In the forearm itself it can be exposed between FCU and FDS (as described above for the median nerve). At the wrist it lies just lateral to the FCU tendon.
In order to trace it into the hand a zigzag incision is made just lateral to the pisiform and the nerve exposed in Guyon’s canal. The sensory branches and nerves to the hypothenar eminence are seen as the nerve divides at the distal end of the canal. The deep motor branch can be followed under the opponens digiti minimi. In the depths of the palm it is best exposed by displacing the flexor tendons of the fingers laterally.
Radial nerve
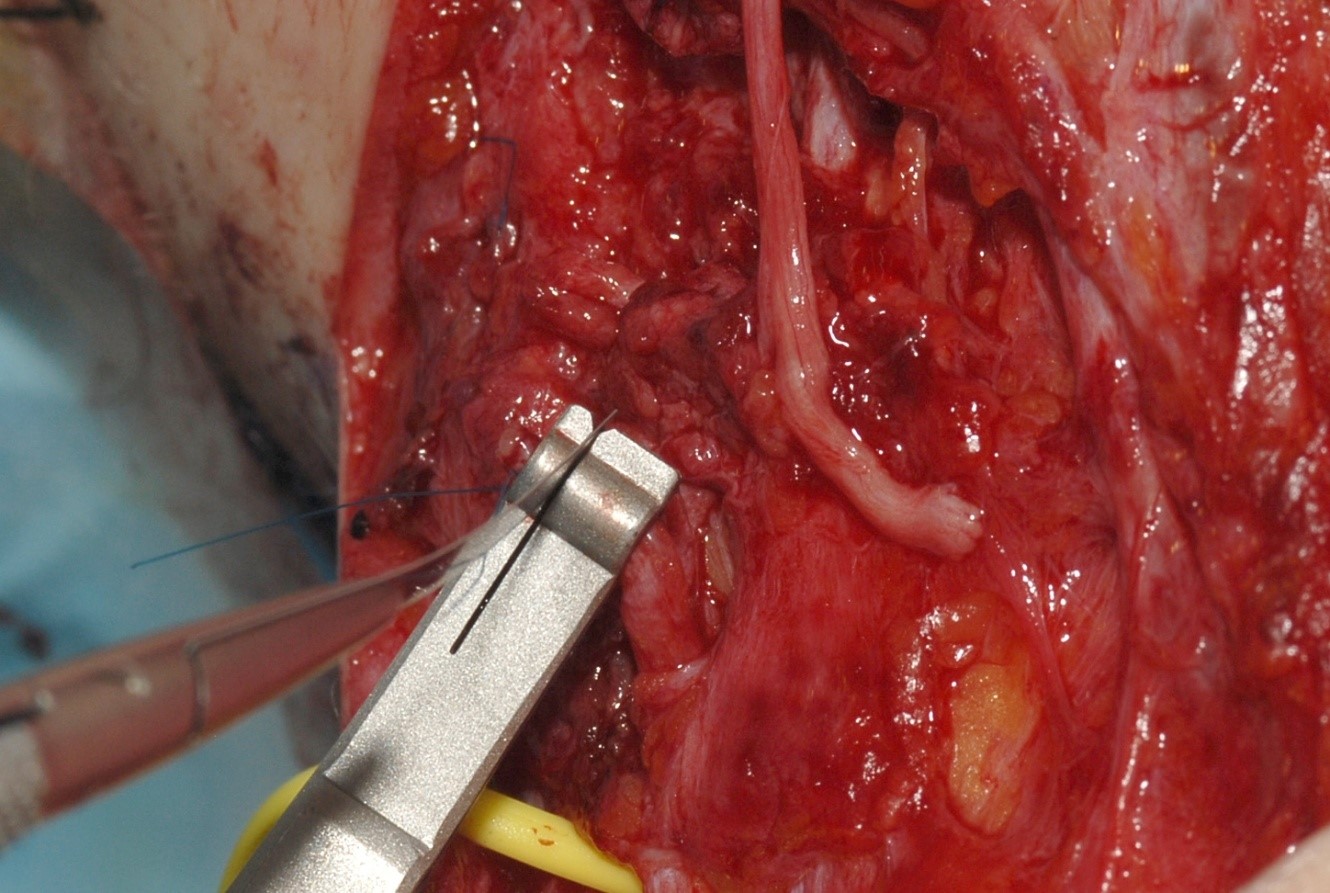
In the middle third of the humerus the radial nerve is most easily exposed through a mid line posterior longitudinal incision. The triceps is split between its lateral and long heads. The nerve is then seen passing obliquely around the spiral groove giving off branches to the triceps muscle. At the junction of the middle and distal thirds of the forearm the nerve penetrates the lateral intermuscular septum and can be exposed through a direct lateral approach.
At the elbow the approach is anterior with the incision being between the brachioradialis muscle and the biceps tendon. It should be S-shaped if crossing the elbow crease. The nerve is identified lying between the brachialis and brachioradialis muscles. In the forearm the superficial sensory branch can be found on the deep surface of brachioradialis until it passes under the muscle to lie subcutaneously on the radial border of the wrist.
The origin of the posterior interosseous nerve (see figure 2) can be seen through the anterior exposure at the elbow.
into posterior interosseousand superficial radial nerves. The sloop on the right is
around the posterior interosseous nerve as it enters supinator.
At the junction of the proximal and middle thirds of the forearm an incision is made on the posterolateral aspect. The plane between the radial wrist extensors (ECRL and ECRB) and extensor digitorum communis (EDC) is developed. The nerve and its branches can then be seen emerging from supinator. Care must be taken during this exposure not to damage the branches which curve superficially to innervate EDC.
Intra-operative neurophysiology
Nerves can be stimulated during an operation in order to test their function. The stimulus can be applied with a sterile bipolar electrode connected to a neurophysiology machine or with a disposable sterile battery powered stimulator, which has a unipolar electrotrode (see figure 3a–b).
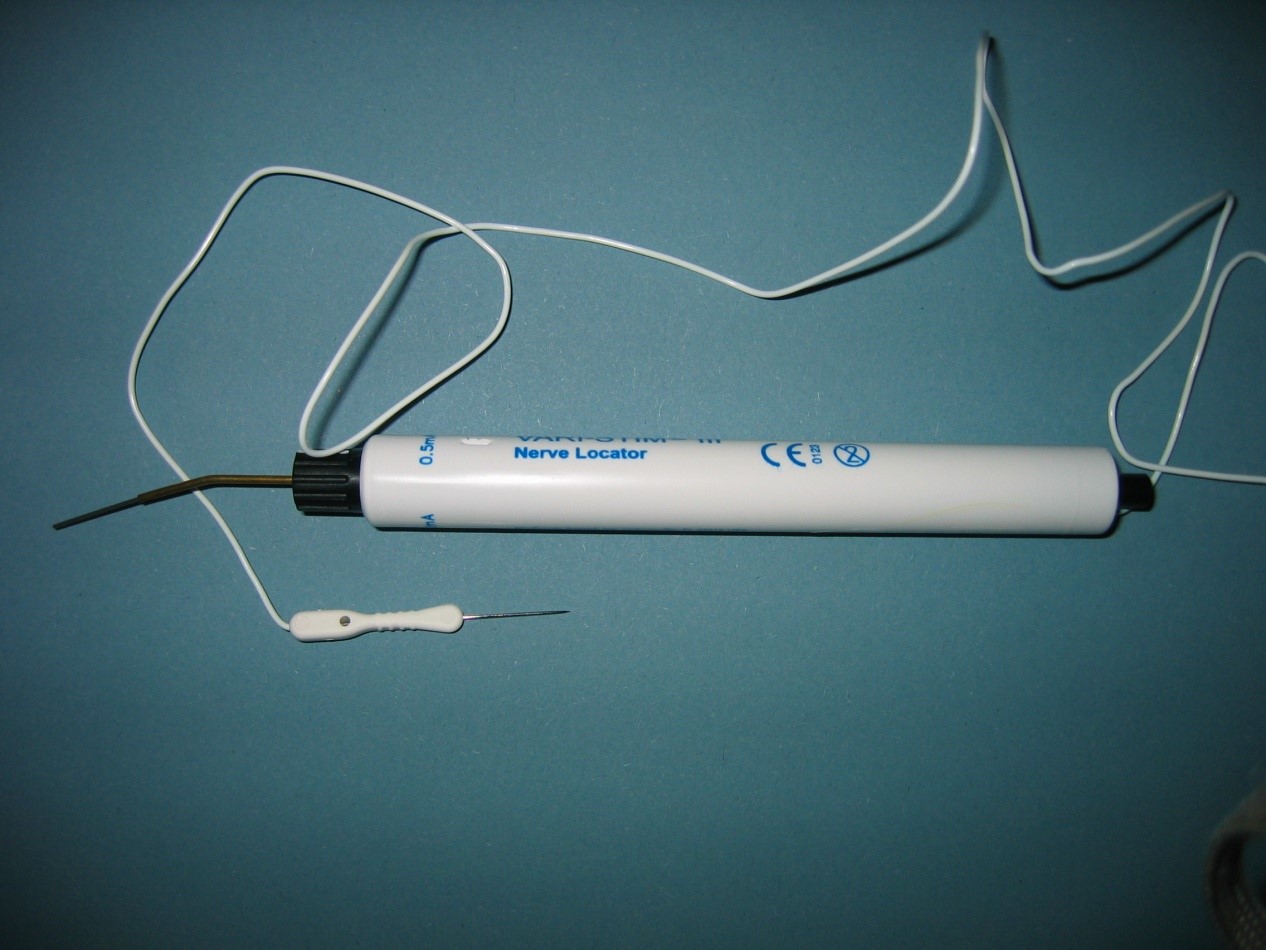
The needleelectrode is placed in adjacent tissue and the probe on the nerve to be tested.
It should be remembered that nerves stop conducting as a result of ischaemia, when a surgical tourniquet has been in place for more than about 30 minutes. When carrying out intraoperative nerve stimulation it also is important to take into account the time from injury. The axons in the distal segment of a degenerating nerve continue to conduct for 2–4 days after the injury. There will therefore be a response to stimulation if operation is carried out within that time. This may be misinterpreted as indicating less severe damage to the nerve such as conduction block if the nerve trunk appears to be in continuity.
Observation of the muscle response after direct stimulation of the nerve is the simplest investigation which gives useful information. If there has been a definite loss of continuity of a nerve trunk when exploring an injury within a few days, stimulation of the distal stump can help to define functional topography of the nerve. Fascicles innervating particular movements can be identified and may be targeted in the reconstruction.
The second type of test is recording of nerve action potentials. This requires a Neurophysiology Unit. Bipolar electrodes are placed on the nerve above and below an area of damage. The stimulus is applied proximal to the lesion and recording made distally. The test is most useful in assessing a lesion in-continuity, when there is no muscle response to stimulation. In this situation, the presence of a nerve action potential shows that axons have regenerated across the lesion in the nerve but not reached the muscles that they innervate [1].
Primary repair of nerves
Direct suture
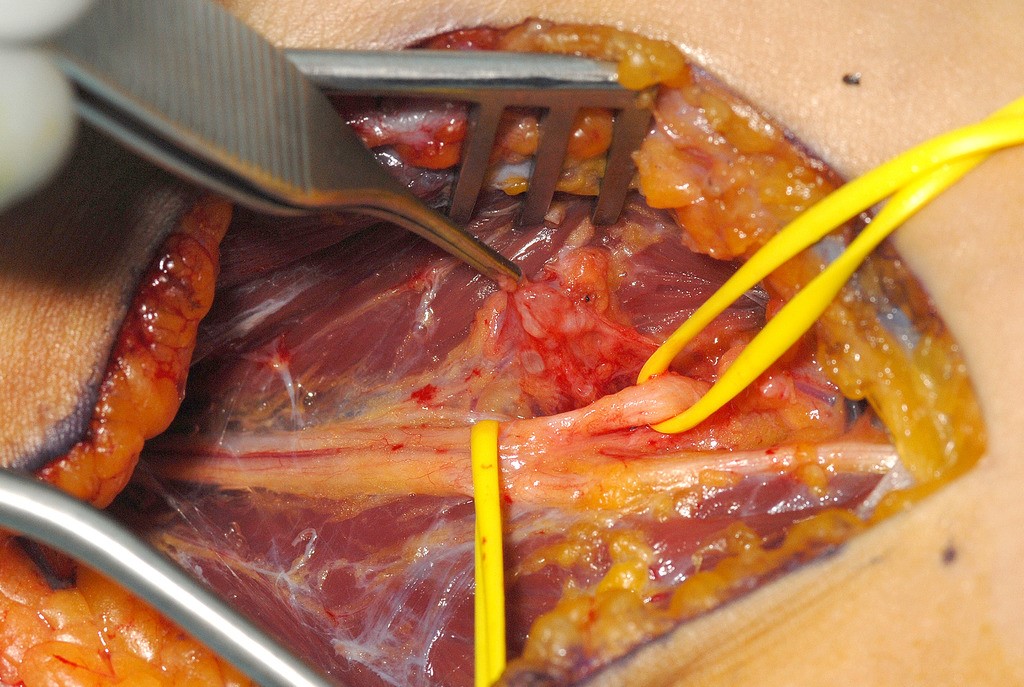
If a nerve has been cleanly divided then it can usually be repaired by direct suture. The wound must be cleaned and adequately debrided. Repair of any other structures damaged by the injury should also be carried out. It must be possible to approximate the nerve ends without, or only minimal, tension. The methods of direct nerve suture include epineurial repair, group fascicular repair and fascicular repair. In epineurial repair sutures are placed around the nerve in the epineurium. The correct alignment of the nerve ends is important. Observation of the fascicular pattern in the two ends of the nerve and blood vessels in the epineurium help with alignment (Figure 4).
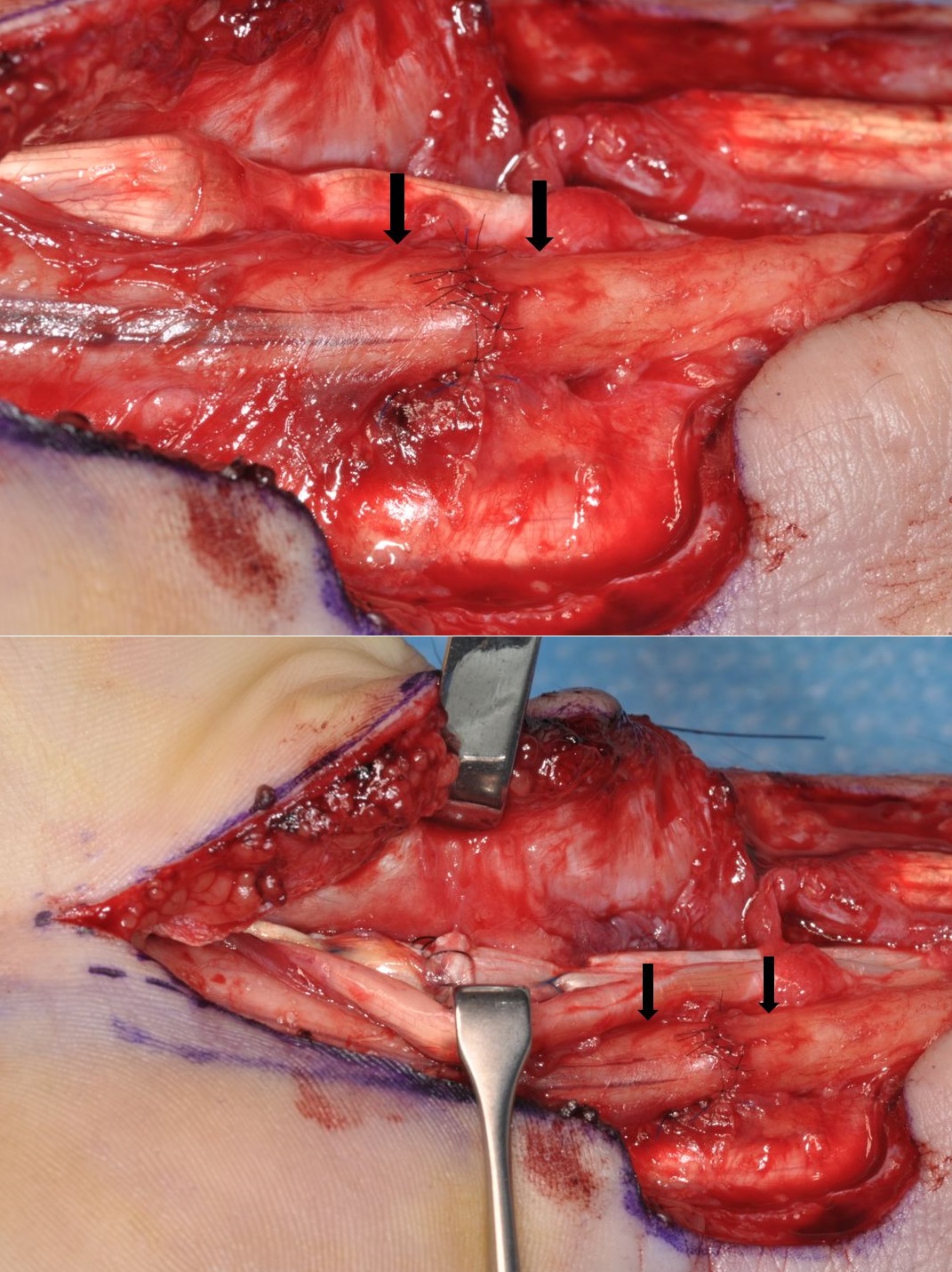
as a result of a laceration on glass. The repair was carried out after a few weeks delay,
but it was still possible to achieve direct suture without excessive tension (→).
The flexor pollicis longus tendon was repaired at the same operation.
Group fascicular or fascicular repair probably doesn’t confer advantage in most situations, except at distal sites where the fascicular bundles are separating to form terminal branches. Surgical trauma should be minimized when separating fascicular bundles.
When possible the nerve ends should be approximated by suturing adjacent tissue, such as mesoneurial attachments. This helps to reduce tension on the repair itself. Typically the deep side of the nerve is sutured first by placing one or two larger gauge sutures. The repair is then completed with smaller sutures working towards the superficial side. Generally 8/0 sutures – are used for epineurial repair of the main nerve trunk in the forearm, with 9/0 or 10/0 sutures for digital nerves. While a “watertight” repair is not necessary there should be sufficient sutures in the epineurium to cover the fascicles of the nerve. The number of sutures may be reduced once the nerve ends have been approximated by using fibrin glue.
Nerve tubes
An alternative type of nerve repair is to place the nerve ends in a tube or conduit. This may be a piece of vein or a synthetic material. Lundborg et al. [2] proposed that encasing the ends of a divided nerve in a tube, leaving a short gap in between, would allow accumulation of neurotrophic factors inside the tube thus encouraging axonal regeneration. It has also been suggested that a semi rigid tube may prevent scar tissue from forming between the nerve ends. In a prospective randomized study comparing the use of silicon tubes for repair of the median and ulnar nerves in the forearm with direct microsurgical suture, Lundborg et al. reported no difference in the quality of nerve recovery. The silicon tubes do not resorb and therefore may need to be removed later. Tubes made from bioabsorbable material have become available including polyglycolic acid, caprolactone, and collagen. It is important that the material should cause as little inflammation as possible. Bioabsorbable glass tubes have also been proposed [3], [4]. The surgical technique, which has been reported by a number of authors, including [5] Agnew, is relatively simple and may be applicable where microsurgical expertise and equipment is not available. The nerve tube should be slightly larger than the diameter of the nerve. The nerve ends, which are freshened, if necessary, are then drawn into the ends of the nerve tube using mattress sutures placed through the wall of the tube.
Complications have been reported, including development of fistulas over the repair following using of caprolactone tubes [6]. Therefore, the quality of soft tissue cover over a nerve conduit is important.
Managing nerve gaps
When there is a ragged laceration of a nerve or a nerve has been ruptured by blunt trauma, then the nerve ends must be trimmed back to healthy fascicles. This is effectively a loss of nerve tissue and leaves a gap in the nerve. Unless the gap is very small direct suture will not be possible (see figure 5).
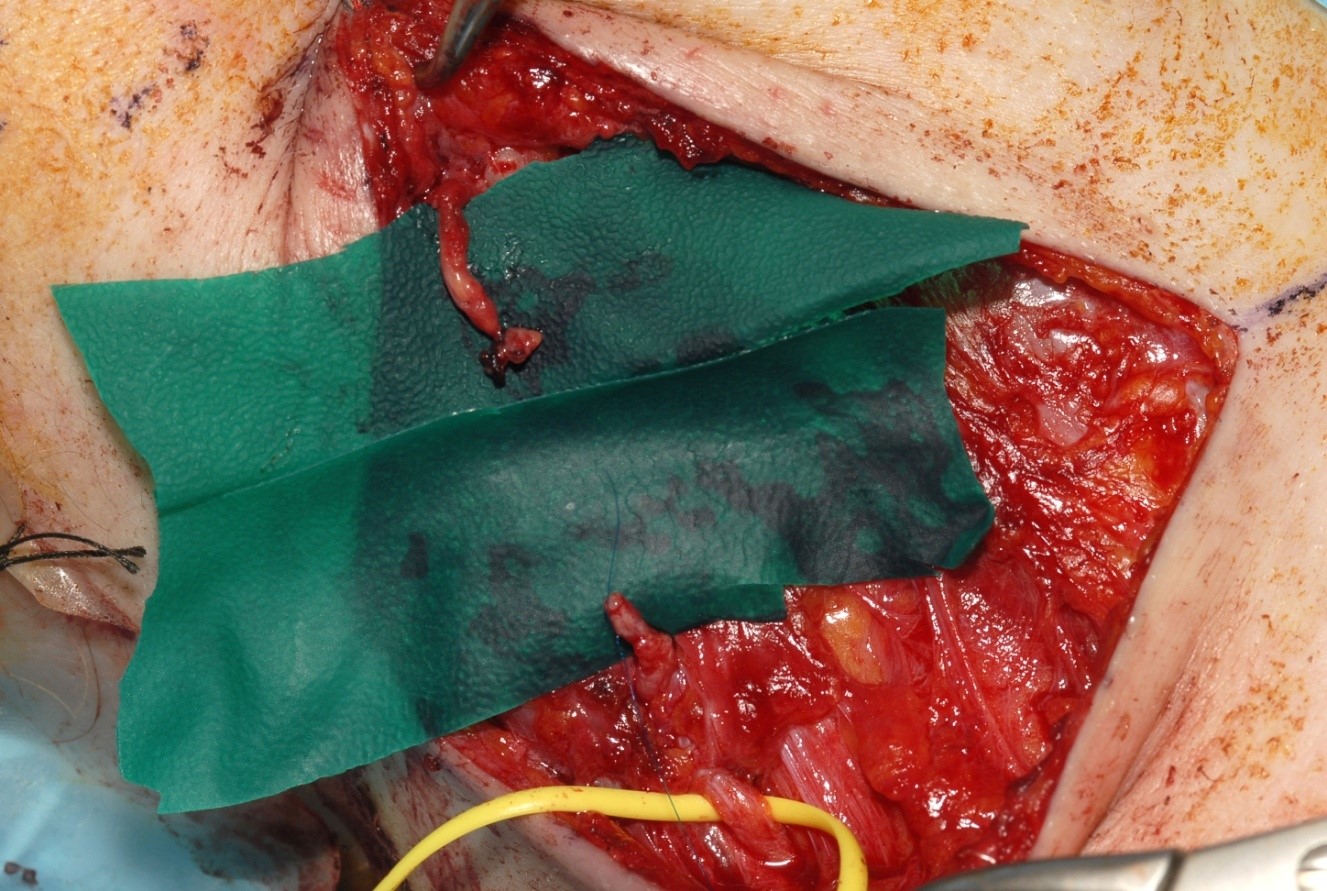
possible without excessive tension.
At some sites mobilisation of the nerve ends together with flexion of adjacent joints may gain some length and allow direct repair. At the elbow the ulnar nerve may be transposed anteriorly. Historically, this approach was utilised during the first half of the 20th century. The limb was splinted with the adjacent joints flexed. After surgery the joints were gradually extended with a series of splints. Some defects of 10 cm or more in nerves were closed using this technique but poor results were noted with these long gaps [7]. The method was investigated experimentally by Highet and Sanders [8]. Stretching the nerve when mobilizing the limb after operation was found to cause extensive degeneration in the proximal stump and fibrosis both in and around the nerve. Other authors [9], [10] have also reported experimental work indicating the deleterious effect of tension at the site of nerve repair. Tension appears to promote connective tissue formation by causing ischaemia at the nerve ends. In general, mobilisation of the nerve and flexion of joints will only enable direct suture to be performed if the gap is less than 2 to 3 cm.
If there are fractures associated with the injury, then in the upper extremity, bone shortening at the fracture site can be considered to enable direct nerve repair. Several centimetres of shortening of the humerus is well tolerated. Bone shortening is routinely carried out with replantation of digits. In the forearm shortening is less well tolerated and it is important to shorten both the radius and the ulna by exactly the same amount. If tension free repair cannot be achieved with the techniques described above then a graft should be performed.
Nerve tubes for short defects
Nerve tubes appear to support regeneration of a nerve across a short gap. This may allow repair of a nerve where trimming of damaged ends has been necessary. Tension at the repair can be avoided by leaving a gap within the tube. McKinnon and Dellon [11] used bioabsorbable polyglycolic acid tubes to repair gaps of 0.5 to 3 cm (mean 1.7 cm) in digital nerves. There was good or excellent sensory recovery in 86% of cases. A prospective randomised study was reported by Weber et al. [12] in which digital nerves were reconstructed with end to end suture or nerve grafts compared with a polyglycolic acid conduit. The mean gap length was 7 mm for the conduit repairs. Recovery in sensation, measured by two-point discrimination, was equal or better for the nerve conduit repairs. Experimental work suggests that the longest gap that can be effectively repaired using a tube repair is about 3 cm according to Mackinnon and Dellon [11]. Repair of longer gaps has been reported using a tube containing a slice of autogenous nerve [13]. Tang et al. [14] reported a similar technique using an autogenous vein graft to repair defects in digital nerves.
In recent years bioabsorbable nerve tubes have gained popularity for repair of digital and other sensory nerves in the hand particularly where there is a small defect and tension free suture is not possible. Their use, however, remains controversial. Cheng [15] reviewed the subjected and concluded that current evidence has not clearly shown that tube repair is better or even equivalent to a nerve graft for a digital nerve defect.
In general, there is a case for considering a tube repair for a short defect in a sensory nerve when harvesting a nerve graft may not be justified. Motor nerves should usually be repaired with a nerve graft.
Non-neural grafts
Grafts of non-neural tissue have been evaluated for repairing defects in nerves. In 1986 Glasby et al. [16] reported the use of freeze-thawed skeletal muscle autografts. These grafts contain a basement membrane framework into which regenerating axons and supporting cells may migrate. The muscle basement membrane is similar in structure to the endoneurial tubes of a nerve, and appears to provide some support for regenerating axons, acting as a neurite promoting factor. Early work showed the muscle grafts to be effective in repairing 1 cm defects in the rat sciatic nerve [16], and encouraging results were reported for human digital nerves and nerves affected by leprosy [17], [18]. However, further experiments showed the grafts to be ineffective in defects of 5 cm or more [19]. These findings together with work on synthetic grafts [20] suggest that grafts or tubes which do not contain live Schwann cells are not able to support nerve regeneration across a gap of more than 2 to 3 cm. Repair of longer gaps has been reported using a tube containing a slice of autogenous nerve [13]. Seeding of grafts or conduits with cultured Schwann cells has been suggested but developments have not yet reached clinical application [20]. There are probably few indications for non-neural grafts at present.
Nerve grafting
The most widely used and currently effective, method of repairing a nerve when direct suture is not possible is nerve grafting. A length of an expendable, usually sensory nerve, from the same individual (autograft) is used to reconstruct a more important nerve. Nerve grafts were attempted in the latter part of the 19th century [21]. Experimental work reported early in the 20th Century [22] showed that nerve regeneration was possible through nerve grafts. Surgeons at the end of the First World War were pessimistic about the clinical applications of nerve grafting. Platt [23] reported the results of 15 nerve grafting operations all to be complete failures. However, there had been considerable delay between injury and operation. Also the grafts were wrapped in sheaths of fascia which we now know hinders revascularisation.
There was a revival of interest in the Second World War. In 1942 Sanders and Young [24] reviewed the subject and showed encouraging results in animals. Nerve grafts are usually free grafts which must revascularize for their constituent cells to survive. The graft provides a pathway for axon regeneration similar to that offered by the distal nerve, except that the size and number of endoneurial tubes can never be identical to the recipient nerve. Cell labelling experiments [25] have shown that the Schwann cells in the nerve graft myelinate regenerating axons rather than cells migrating into the graft. The nerve graft is therefore more than just a framework for axon regeneration.
Tarlov and Epstein [26] showed that free nerve grafts are revascularized with vessels entering from both ends and the surrounding tissue bed within a few days. Clinical observations suggest, that while small nerves are effectively revascularized, a graft from a large diameter nerve tends to undergo central necrosis and fibrosis.
Much clinical work performed during the Second World War by Seddon and others fully established nerve grafting as a technique. In 1947 Seddon [27] reported the results of a series of 58 cases in which extensive gaps in nerves were repaired with grafts. In 20 (38.5%) recovery was as good as that seen after a satisfactory direct suture of the same nerve. Recovery was proceeding well or there was partial recovery in another 15 cases, so that the operation was of value in 67.3%. In 1955 Brooks [28] reported similar results from a series of 93 patients. Most nerve grafts are thin cutaneous nerves. For repair of larger diameter nerves the operation of cable grafting, which had been suggested by Kilvington [22], was devised. In this technique, multiple strands of the graft are used in parallel to build up the required cross-section of the nerve being reconstructed. Although the outcome of nerve grafts appears to deteriorate with increasing length, defects of 15 to 20 cm can be successfully bridged in some nerves.
Further developments were made by Millesi [9], [29] , whose results suggested that nerve grafting is superior to suture under tension even when quite short lengths of nerve are lost. He introduced the concept of interfascicular nerve grafting, where fascicles at each end of a divided nerve are matched as closely as possible. After identifying corresponding fascicles at the two cut nerve ends appropriately chosen nerve autografts are used to connect them. He also emphasised the importance of avoiding any tension in a nerve graft repair. The technique, as it was originally described, involved resection of a cuff of epineurium from each nerve stump and isolating major fascicular bundles. The additional surgical trauma has to be weighed against the advantages of matching fascicles.
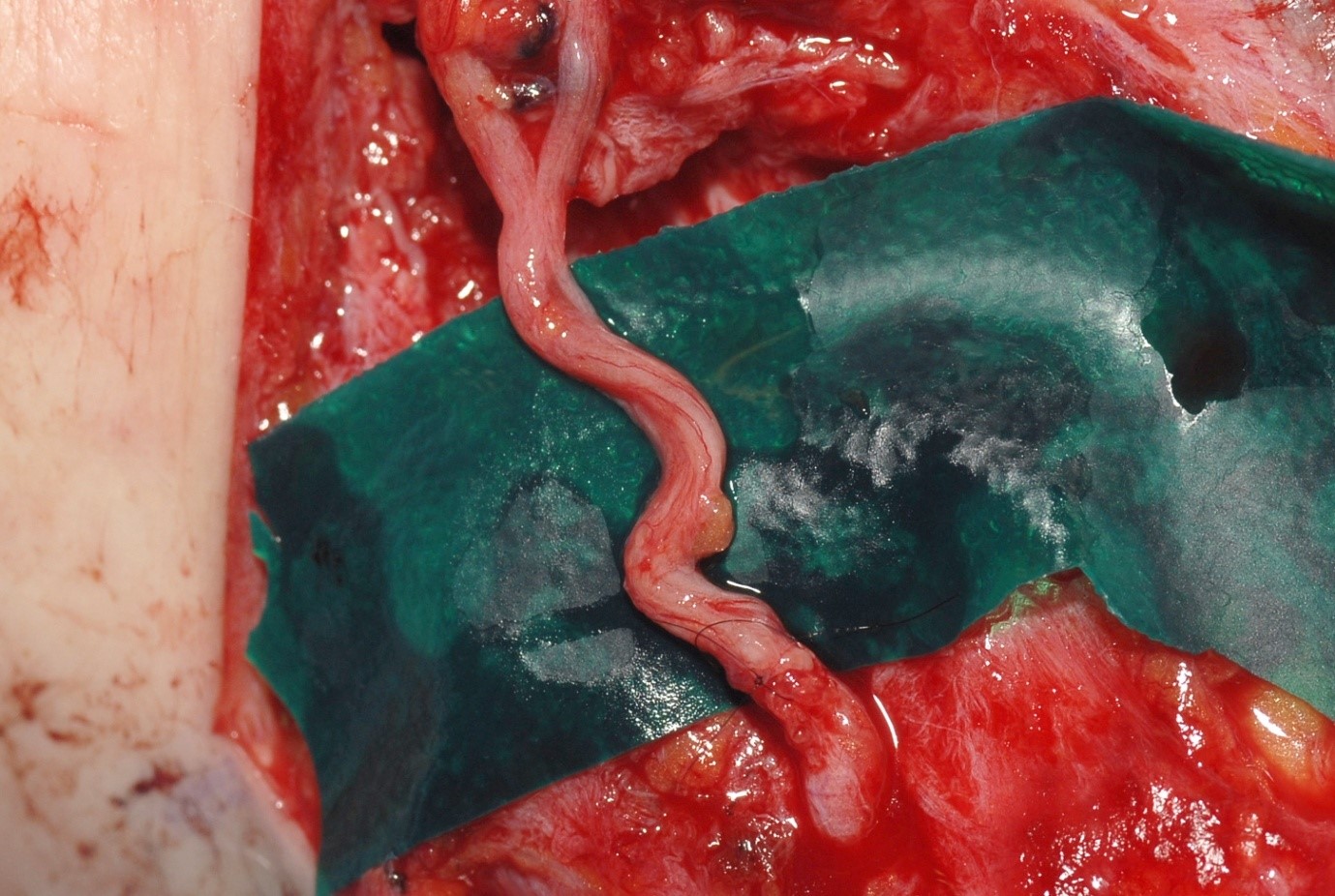
Vascularized nerve grafts
The observation that the blood-supply to a nerve graft is important in its outcome led to the idea of vascularised nerve grafts. In 1950, St. Clair Strange [30] reported the use of a pedicled nerve graft in a patient who had gaps in both the median and ulnar nerves. The median nerve was repaired using a graft from the ulnar nerve. In the 1970s Taylor and Ham [31] reported free microvascular transfer of the superficial radial nerve with the radial artery and its venae comitantes. The ulnar nerve has been used to repair the upper part of the brachial plexus when the C8 and T1 roots are known to be irreparably damaged [32]. Both experimental evidence [33] and clinical results have not shown a clear advantage for vascularised nerve grafts in most circumstances. However, they may be useful where the recipient tissue bed is scarred, therefore not providing a good environment for revascularisation of a free graft.
Surgical technique
After adequate exposure, the two ends of the nerves are prepared by trimming until normal nerve tissue is seen. The grafts should lie in a vascularised tissue bed. In order to avoid any tension the grafts should be at least 10% longer than the nerve gap. If possible, equivalent fascicles in the two ends should be identified and grafts connected accordingly. However, this can usually only be achieved if a short length of nerves has been lost or at distal sites where the nerve is branching. It can be easier to identify individual fascicular bundles if there are transected at the different levels.
One or two sutures are inserted to hold each strand of nerve graft (8/0 or 9/0). The sutures pass through the epineurium of the graft and the epineurium of the nerve or the perineurium of a fascicular bundle. Further strength can sometimes be obtained by placing side to side sutures away from the point of coaptation. The repair is often reinforced with fibrin glue. However, this should only cover the point of coaptation rather than the whole length of the graft.
Donor nerves for grafting
The sural nerve is the most widely used donor for nerve grafting. The sural nerve provides up to 45 cm of graft, is usually easy to harvest, and donor site morbidity has been reported as low [34]. However, it can be difficult to obtain the graft when a patient is supine on the operating table. When operating on the upper limb a new sensory deficit together with incisions and scarring are created on a non injured limb. Staniforth and Fisher [35] reported calf tenderness in 42% of patients and a significant painful neuroma in 16% of patients who had had harvesting of the sural nerve. Other donor nerves include the medial cutaneous nerve of the forearm (MCNF), the lateral cutaneous nerve of the forearm (LCNF), the superficial radial nerve (SRL) [36], [37], and the posterior interosseous nerve (PIN).
It is attractive to utilise donor nerves from the same limb or the same surgical field when possible. In addition, the sensory nerves which have already been affected by the injury may be suitable for grafting. For example, if a nerve affected by a more proximal nerve lesion is harvested then there will be no new sensory deficit created. The lateral cutaneous nerve of the forearm may be used for grafting when there has been an injury to the musculocutaneous nerve or to the C5 and C6 roots of the brachial plexus. The superficial radial nerve should usually only be harvested when there has been a more proximal injury to the radial nerve. In these situations useful sensory recovery is unlikely to have occurred in the donor nerves if they had not been used for grafting. There are occasionally other possibilities such as, using the dorsal cutaneous branch of the ulnar nerve as a graft if there is a short defect more proximally in the ulnar nerve.
Since the nerve used as a donor for grafting has been divided, the axons within the nerve will undergo Wallerian degeneration. The subsequent proliferation of Schwann cell and production of neurotrophic factors is likely to favour regeneration of axons in the recipient nerve. Indeed Jibain et al. [38] showed in an experimental model that early or delayed repair with a nerve graft reduces loss of motor neurons. If there has been a delay between injury and operation then the graft may have less biological activity with lower levels of growth factors and consequently be less effective. While it has been difficult to carry out robust comparison between cases, the author’s observations of clinical cases suggest that there is no difference in outcome between grafts obtained from injured nerves compared with those from un-injured nerves, even when there has been a delay between injury and operation.
A painful neuroma at the proximal stump of a nerve that has been harvested for grafting is a potential complication. This may be minimised by dividing the nerve at a deeply placed site rather than where it is subcutaneous. The technique for harvesting specific nerves is described below.
Sural nerve
Before harvesting of the sural nerve patients should be warned of the reduction of sensation on the lateral border of the foot. However, this rarely causes a functional problem. The procedure is usually carried out with a thigh tourniquet in place. Depending on the amount of graft required the nerve may be harvested from one or both legs. A skin incision is first made at the ankle half way between the lateral malleolus and the Achilles tendon. The nerve is identified at this level. Gentle traction is then applied to the nerve while palpating along the course of the nerve proximally. This allows the position of the nerve to be identified. A further 2–3 cm incision is then made at the mid calf level and the nerve again identified and isolated with a rubber sloop. Finally a transverse incision is made in the skin crease over the popliteal fossa. Here the sural nerve can be identified lying between the two heads of gastrocnemius adjacent to the sural artery and vein. Tugging gently on the nerve in each incision can confirm that the same nerve has been identified. The sural nerve is then divided as far proximally as possible allowing the proximal stump to retract deeply into the popliteal fossa. Traction is then applied to the nerve in the mid calf incision allowing it to be delivered into that incision and then into the distal incision in a similar manner. It is divided as far distally as possible. If difficulty is encountered removing the nerve through these incisions then additional short incisions may be needed to free adhesions. Particular difficulty may be encountered if there is a communicating branch from the peroneal nerve in the proximal calf. This will prevent harvesting of the sural nerve unless it is identified and divided.
Lateral cutaneous nerve of the forearm (25 cm, C5, C6, and C7)
The lateral cutaneous nerve of the forearm can usually be harvested between mid arm and mid forearm levels providing about 25 cm of graft. A longitudinal incision is made on the anterior aspect of the proximal forearm starting just lateral to the biceps tendon. The nerve can be identified passing subcutaneously on the lateral side of the biceps tendon. It can then be traced distally until it divides into small branches. If more graft is required a medial incision is made in the mid forearm and the plane developed deep to the biceps muscle where the musculocutaneous nerve and its branches can be identified. After dividing the LCNF distally it is then delivered into this proximal incision and mobilised as far proximally as the last motor branch of the musculocutaneous nerve, which is usually the branch to brachialis. It is then divided.
Medial cutaneous nerve of the forearm (30 cm, C8, T1)
The MCNF can be harvested from the full length of the upper arm. A longitudinal or transverse incision is made a little in front of the medial epicondyle and the nerve identified at this level. A further incision is then made on the medial aspect of the arm just below the axilla with the nerve being identified as far proximally as possible and then divided. It can usually then be delivered into the distal incision. However there is variations in branching of the nerve which may necessitate further mobilisation before harvesting. It is closely related to the basilic vein. If the infraclavicular brachial plexus has been exposed through a delto-pectoral incision then the nerve may be traced even more proximally and divided where it branches from the medial cord.
Superficial radial nerve (SRN, 25 cm, C6)
The superficial radial nerve, when appropriate, can be harvested between the wrist and the elbow. An incision is made over the radial styloid and the nerve identified. An incision is then made on the proximal forearm medial to brachioradialis. The nerve is identified on the deep surface of the brachioradialis muscle. It is divided distally and then delivered into the proximal incision. It may be then traced a bit further proximally carefully separating it from the posterior interosseous nerve and motor branches to the wrist extensors, before dividing it.
Posterior interosseous nerve (PIN)
The terminal branch of the posterior interosseous nerve, which provides sensory innervation to the wrist joint, may be used for reconstruction of digital nerves. An incision is made on the dorsum of the distal forearm and the fourth tendon compartment is opened proximal to the extensor retinaculum. The posterior interosseous nerve can be identified in the floor of the 4th compartment and then is traced proximally until the last motor branch, usually to EPL, is encountered. It is then divided and removed. Typically 5 or 6 cm of this slender nerve can be obtained.
Secondary repair of nerves
Delayed exploration and repair of a nerve may be carried out following open injuries, when primary repair has not been possible, or after closed blunt injury to the nerve. Substantial exposure is usually necessary. There is likely to be scarring at the site of injury. Therefore the nerve should be exposed first in normal tissue both proximally and distally. Dissection is then made along the course of the nerve towards the zone of injury. When a nerve has been divided then a neuroma will have formed on the proximal stump and there is usually a swelling on the distal stump. The nerves ends are mobilised. It is important repair is made after trimming the ends back to healthy unscarred fascicles. It is only when this has been done that the gap in the nerve will be evident. Even after a clean laceration there is retraction of the nerve ends if there has been delay of more than one or two weeks after the injury. Repair should then be undertaken using the principles described in the section on managing nerve gaps. Very often a nerve graft is required for secondary repair of the nerve.
Blunt injuries
If there has been a violent stretching of the nerve then there may be clear loss of continuity with swelling on the proximal and distal stumps. In this situation there is often a considerable defect of 10 cm of more, after trimming back to healthy fascicles. However, in less severe injuries the nerve may still be in continuity and careful assessment is required to predict whether the lesion is likely to recover spontaneously or has a better chance after surgical repair. Assessment is based both on the appearance of the nerve and response to stimulation.
The appearance of the nerve is important. If there is no swelling or constriction then this suggests axonotmesis or Sunderland second degree injury. Swelling of the nerve indicates a more severe injury and the presence of a double bulb usually indicates a Sunderland 4th degree injury where fascicles have ruptured leaving only a fibrotic epineurium remaining. This type of lesion will not recover spontaneously. When in doubt the epineurium can be opened longitudinally and fascicles traced to establish whether there is continuity of these across the injured segment.
Electrical stimulation is first applied proximal to the lesion. If there is any response in innervated muscles then in general there is a good chance of continuing recovery in the nerve. If there is no response to stimulation, providing there has been a delay of two or more months since the injury, then recording of a nerve action potential may give useful information (see also: Intra-operative neurophysiology). If a nerve action potential can be recorded this indicates that axons have regenerated across the lesion and there is a good chance of subsequent recovery in the nerve. If no action potential is recordable then the chance of spontaneous recovery is lower.
Despite these measures, assessment of some nerve lesions remains difficult. Overall, it is usually best to proceed to repair with a nerve graft providing there is a focal lesion identified in the nerve and there is no response to stimulation and no nerve action potential, providing 2–3 months or more has elapsed since the injury.
Neurolysis
Neurolysis is a term given to a procedure where a nerve is released from compression or scar tissue. External neurolysis involves freeing the nerve from external compression such as may occur in the carpal canal or as a result of a displaced fracture. The epineurium of the nerve is left intact. Providing the compression does not recur external neurolysis can be an effective procedure allowing the recovery of the nerve to proceed. If the epineurium is opened and fascicles are dissected from intraneural scar tissue, this is termed internal neurolysis. Unfortunately, recurrence of scarring is very likely following such a procedure and there isn’t clear evidence that it will lead to improvement in the function of the nerve.
Although neurolysis is often performed during secondary exploration of an injured nerve, partly in the process of defining the injury, it is doubtful whether the procedure changes the outcome unless there is has been clear external compression of the nerve [36].
Technique of nerve graft repair
If it is decided, on delayed exploration, to carry out nerve graft repair of a severe blunt injury to the nerve or even after laceration of the nerve, then it is important that normal fascicles are identified proximal and distal to the lesion and that nerve grafts bridge the intervening segment. This may be achieved by trimming the ends back until fascicles with a normal appearance are found. Specimens can be subjected to frozen section histological examination to confirm the nerve architecture and degree of fibrosis.
Alternatively the nerve ends are not mobilised. Instead a longitudinal incision is made through the epineurium proximally and distally. The fascicles are then divided within the epineurium and nerve grafts placed bridging the damaged segment. This technique has the advantage of preventing any retraction of the nerve ends and therefore reducing tension on the repair. As well as sutures at the end of each nerve grafts further sutures can be placed side to side between the epineurium of the nerve and the grafts therefore adding strength to the repair.
Nerve transfers
A nerve transfer is a procedure where a functioning expendable nerve is connected to re-innervate a more important nerve. This type of procedure may enable re-innervation of the distal stump of an injured nerve when the proximal nerve is not available or the gap is not amenable to reconstruction. In some situations a nerve transfer may represent a more reliable method of re-innervation of the nerve than reconstruction of the nerve itself.
The donor nerve has to be selected carefully to avoid any significant deficit from its use. Whenever possible nerve transfers should be joined by direct suture and an inter-positional graft avoided. This dictates the donor nerve being closely related to the recipient nerve.
Nerve transfers are most often carried out for restoration of motor function. The function produced as a result of a nerve transfer cannot be independent of that of the donor nerve. Therefore problems may be encountered with co-ordination of movement and co-contraction. The situation may be optimised by selecting donor nerves whose normal function is synergistic with the function to be restored. It is unlikely that a single nerve transfer can restore more than one movement. Therefore, if multiple movements need to be reconstructed then more than one donor nerve should be used.
Nerve transfer was suggested following experimental work as far back at the 19th Century. Kilvington [40], [41] reviewed the subject and carried out further experimental work. The technique has gained considerable popularity over the last 20–30 years particularly for reconstruction of the brachial plexus. Nerve transfers have been described for re-innervation of nerves more distally in the forearm and hand. For example, radial nerve palsy may be treated by transfer of the flexor digitorum superficialis branch of the median nerve to the extensor carpi radialis brevis nerve for wrist extension and the flexor carpi radialis branch of the median nerve to the postero-interosseous nerve of the finger and thumb extension [42]. Intrinsic function in the hand after ulnar nerve injury may be restored by transferring the terminal branch of the anterior interosseous nerve to the motor branch fascicle of the ulnar nerve [43].
Sensory nerve transfers have also been described in the hand. A nerve which supplies a less important area of sensation may be transferred to one innervating a more important area or skin. For example, the ulnar nerve branch to the 4th web space may be transferred to the median nerve branches to the thumb and 1st web space in the event of median nerve injury. While sensation may be restored it is unlikely that this is good quality or well localised.
End-to-side repair
Another technique which has been proposed when the proximal stump of a damaged nerve is not available is end-to-side repair. The distal stump of the damaged nerve is sutured into an epineurial or perineurial opening made in an adjacent nerve. It is thought that the neurotrophic influence of the recipient nerve can induce collateral sprouting of axons in the donor nerve which may then grow into the recipient nerve. Vertibo et al. [44] reported success with the technique in a rat model. However, experimental work carried out in a large animal model (sheep) showed very poor regeneration after end-to-side repair of forearm nerves [45]. Other recent evidence suggests that limited collateral sprouting of sensory fibres is possible after opening the perineurium [46], but motor axons will only regenerate if there is injury to axons in the donor nerve.
Pienaar et al. [47] reported the outcome in 10 patients who had undergone end-to-side repair. None demonstrated any objective motor recovery. Four had some recovery of protective sensation. The authors had abandoned the technique. Therefore current evidence suggests that surgical applications of end-to-side repair appear to be limited. The technique has been recommended in preference to nerve transfer for sensory nerves as sensation is preserved in the donor nerve territory [43].
References
[1] Kline DG, Hudson AR. Nerve Injuries. Operative results for major nerve injuries, entrapments, and tumors. Philadelphia: W.B. Saunders; 1995.[2] Lundborg G, Rosén B, Dahlin L, Danielsen N, Holmberg J. Tubular versus conventional repair of median and ulnar nerves in the human forearm: early results from a prospective, randomized, clinical study. J Hand Surg Am. 1997 Jan;22(1):99-106. DOI: 10.1016/S0363-5023(05)80188-1
[3] Lenihan DV, Carter AJ, Gilchrist T, Healy DM, Miller IA, Myles LM, Glasby MA. Biodegradable controlled release glass in the repair of peripheral nerve injuries. J Hand Surg Br. 1998 Oct;23(5):588-93. DOI: 10.1016/S0266-7681(98)80007-7
[4] Jeans LA, Gilchrist T, Healy D. Peripheral nerve repair by means of a flexible biodegradable glass fibre wrap: a comparison with microsurgical epineurial repair. J Plast Reconstr Aesthet Surg. 2007;60(12):1302-8. DOI: 10.1016/j.bjps.2006.06.014
[5] Agnew SP, Dumanian GA. Technical use of synthetic conduits for nerve repair. J Hand Surg Am. 2010 May;35(5):838-41. DOI: 10.1016/j.jhsa.2010.02.025
[6] Chiriac S, Facca S, Diaconu M, Gouzou S, Liverneaux P. Experience of using the bioresorbable copolyester poly(DL-lactide-ε-caprolactone) nerve conduit guide Neurolac™ for nerve repair in peripheral nerve defects: report on a series of 28 lesions. J Hand Surg Eur Vol. 2012 May;37(4):342-9. DOI: 10.1177/1753193411422685.
[7] Highet WB, Holmes W. Traction injuries to the lateral popliteal nerve and traction injuries to peripheral nerves after suture. Br J Surg. 1943;30:212-33. DOI: 10.1002/bjs.18003011906
[8] Highet WB, Sanders FK. The effects of stretching nerves after suture. Br J Surg. 1943;30:355-69. DOI: 10.1002/bjs.18003012012
[9] Millesi H, Meissl G, Berger A. The interfascicular nerve-grafting of the median and ulnar nerves. J Bone Joint Surg Am. 1972 Jun;54(4):727-50. DOI: 10.2106/00004623-197254040-00004
[10] Terzis J, Faibisoff B, Williams B. The nerve gap: suture under tension vs. graft. Plast Reconstr Surg. 1975 Aug;56(2):166-70. DOI: 10.1097/00006534-197508000-00008
[11] Mackinnon SE, Dellon AL. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast Reconstr Surg. 1990 Mar;85(3):419-24. DOI: 10.1097/00006534-199003000-00015
[12] Weber RA, Breidenbach WC, Brown RE, Jabaley ME, Mass DP. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000 Oct;106(5):1036-45; discussion 1046-8. DOI: 10.1097/00006534-200010000-00013
[13] Hung V, Dellon AL. Reconstruction of a 4-cm human median nerve gap by including an autogenous nerve slice in a bioabsorbable nerve conduit: case report. J Hand Surg Am. 2008 Mar;33(3):313-5. DOI: 10.1016/j.jhsa.2007.12.008
[14] Tang JB, Gu YQ, Song YS. Repair of digital nerve defect with autogenous vein graft during flexor tendon surgery in zone 2. J Hand Surg Br. 1993 Aug;18(4):449-53. DOI: 10.1016/0266-7681(93)90144-5
[15] Cheng CJ. Synthetic nerve conduits for digital nerve reconstruction. J Hand Surg Am. 2009 Nov;34(9):1718-21. DOI: 10.1016/j.jhsa.2009.07.017
[16] Glasby MA, Gschmeissner S, Hitchcock RJ, Huang CL. Regeneration of the sciatic nerve in rats. The effect of muscle basement membrane. J Bone Joint Surg Br. 1986 Nov;68(5):829-33.
[17] Norris RW, Glasby MA, Gattuso JM, Bowden RE. Peripheral nerve repair in humans using muscle autografts. A new technique. J Bone Joint Surg Br. 1988 Aug;70(4):530-3.
[18] Pereira JH, Palande DD, Subramanian A, Narayanakumar TS, Curtis J, Turk JL. Denatured autologous muscle graft in leprosy. Lancet. 1991 Nov 16;338(8777):1239-40. DOI: 10.1016/0140-6736(91)92105-B
[19] Hems TEJ, Glasby MA. The limit of graft length in the experimental use of muscle grafts for nerve repair. J Hand Surg Br. 1993 Apr;18(2):165-70. DOI: 10.1016/0266-7681(93)90097-Y
[20] de Ruiter GC, Malessy MJ, Yaszemski MJ, Windebank AJ, Spinner RJ. Designing ideal conduits for peripheral nerve repair. Neurosurg Focus. 2009 Feb;26(2):E5. DOI: 10.3171/FOC.2009.26.2.E5
[21] Dellon ES, Dellon AL. The first nerve graft, Vulpian, and the nineteenth century neural regeneration controversy. J Hand Surg Am. 1993;18(2):369-72. DOI:10.1016/0363-5023(93)90378-G
[22] Kilvington B. Report CV. An investigation on the regeneration of nerves with regard to surgical treatment of certain paralyses. Br Med J. 1908 Jun;1(2476):1414-9.
[23] Platt H. On the results of bridging gaps in injured nerve trunks by autogenous fascial tubulization and autogenous nerve grafts. Br J Surg. 1919;7:384-9. DOI: 10.1002/bjs.1800072709
[24] Sanders FK, Young JZ. The degeneration and re-innervation of grafted nerves. J Anat. 1942 Jan;76(Pt 2):143-166.7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1252699/
[25] Aguayo AJ, Epps J, Charron L, Bray GM. Multipotentiality of Schwann cells in cross-anastomosed and grafted myelinated and unmyelinated nerves: quantitative microscopy and radioautography. Brain Res. 1976 Mar 5;104(1):1-20. DOI: 10.1016/0006-8993(76)90643-0
[26] Tarlov IM, Epstein JA. Nerve grafts: The importance of an adequate blood supply. J Neurosurg. 1945;2:49-71. DOI: 10.3171/jns.1945.2.1.0049
[27] Seddon HJ. The use of autogenous grafts for the repair of large gaps in peripheral nerves. Br J Surg. 1947 Oct;35(138):151-67. DOI: 10.1002/bjs.18003513808
[28] Brooks D. The place of nerve-grafting in orthopaedic surgery. J Bone Joint Surg Am. 1955 Apr;37-A(2):299-305. DOI: 10.2106/00004623-195537020-00008
[29] Millesi H, Meissl G, Berger A. Further experience with interfascicular grafting of the median, ulnar, and radial nerves. J Bone Joint Surg Am. 1976 Mar;58(2):209-18. DOI: 10.2106/00004623-197658020-00008
[30] Strange FG. Case report on pedicled nerve-graft. Br J Surg. 1950 Jan;37(147):331-3. DOI: 10.1002/bjs.18003714720
[31] Taylor GI, Ham FJ. The free vascularized nerve graft. A further experimental and clinical application of microvascular techniques. Plast Reconstr Surg. 1976 Apr;57(4):413-26. DOI: 10.1097/00006534-197604000-00001
[32] Bonney G, Birch R, Jamieson AM, Eames RA. Experience with vascularized nerve grafts. Clin Plast Surg. 1984 Jan;11(1):137-42.
[33] Hems TEJ, Glasby MA. Comparison of different methods of repair of long peripheral nerve defects: An experimental study. Br J Plast Surg. 1992;45:497-502. DOI: 10.1016/0007-1226(92)90141-J
[34] Miloro M, Stoner JA. Subjective outcomes following sural nerve harvest. J Oral Maxillofac Surg. 2005;63:1150-4. DOI: 10.1016/j.joms.2005.04.031
[35] Staniforth P, Fisher TR. The effects of sural nerve excision in autogenous nerve grafting. Hand. 1978 Jun;10(2):187-90. DOI: 10.1016/S0072-968X(78)80012-6
[36] Birch R. Nerve Repair. In: Green DP, Wolfe SW, Pederson WC, Hotchkiss RN, Wolfe WS, editors. Green's Operative Hand Surgery. 5th ed. Philadelphia: Elsevier Churchill Livingstone; 2005. p. 1075.
[37] Birch R. Surgical Disorders of the Peripheral Nerves. London: Springer-Verlag; 2011. DOI: 10.1007/978-1-84882-108-8
[38] Jivan S, Novikova LN, Wiberg M, Novikov LN. The effects of delayed nerve repair on neuronal survival and axonal regeneration after seventh cervical spinal nerve axotomy in adult rats. Exp Brain Res. 2006 Apr;170(2):245-54. DOI: 10.1007/s00221-005-0207-7
[39] Reissis N, Stirrat A, Manek S, Dunkerton M. The terminal branch of posterior interosseous nerve: a useful donor for digital nerve grafting. J Hand Surg Br. 1992 Dec;17(6):638-40. DOI: 10.1016/0266-7681(92)90190-D
[40] Kilvington B. An investigation of the regeneration of nerves, with a view to the surgical treatment of certain paralyses. Br Med J. 1905 Apr 29;1(2313):935-40. DOI: 10.1136/bmj.1.2313.935
[41] Glasby MA, Hems TEJ. Basil Kilvington. Unknown pioneer of peripheral nerve repair. J Hand Surg Br. 1993 Aug;18(4):461-4. DOI: 10.1016/0266-7681(93)90146-7
[42] Tung TH, Mackinnon SE. Nerve transfers: indications, techniques, and outcomes. J Hand Surg Am. 2010 Feb;35(2):332-41. DOI: 10.1016/j.jhsa.2009.12.002
[43] Novak CB, Mackinnon SE. Distal anterior interosseous nerve transfer to the deep motor branch of the ulnar nerve for reconstruction of high ulnar nerve injuries. J Reconstr Microsurg. 2002 Aug;18(6):459-64. DOI: 10.1055/s-2002-33326
[44] Viterbo F, Trindade JC, Hoshino K, Mazzoni Neto A. End-to-side neurorrhaphy with removal of the epineurial sheath: an experimental study in rats. Plast Reconstr Surg. 1994 Dec;94(7):1038-47. DOI: 10.1097/00006534-199412000-00019
[45] Kettle SJ, Starritt NE, Glasby MA, Hems TE. End-to-side nerve repair in a large animal model: how does it compare with conventional methods of nerve repair? J Hand Surg Eur Vol. 2013 Feb;38(2):192-202. DOI: 10.1177/1753193412445119
[46] Hayashi A, Pannucci C, Moradzadeh A, Kawamura D, Magill C, Hunter DA, Tong AY, Parsadanian A, Mackinnon SE, Myckatyn TM. Axotomy or compression is required for axonal sprouting following end-to-side neurorrhaphy. Exp Neurol. 2008 Jun;211(2):539-50. DOI: 10.1016/j.expneurol.2008.02.031
[47] Pienaar C, Swan MC, De Jager W, Solomons M. Clinical experience with end-to-side nerve transfer. J Hand Surg Br. 2004 Oct;29(5):438-43. DOI: 10.1016/J.JHSB.2004.03.006



