The stiff proximal interphalangeal joint
1 Department of Hand Surgery, University Hospital of Wales, Cardiff, United Kingdom
Introduction
One of the major challenges, if not the major challenge, in the management of Dupuytren’s Disease is correction of the proximal interphalangeal joint contracture and maintenance of this correction. In 1831 Dupuytren himself [1] recognized the difficulty in straightening the proximal interphalangeal joint. Despite this, much of the early literature focused on Dupuytren’s Disease in the palm [2]. As is well known, in contrast to the metacarpophalangeal joint (MCPJ), the proximal interphalangeal joint (PIPJ) is a less tolerant joint. The correction of this joint, is more difficult to achieve and is more difficult to maintain.
There are a number of factors that contribute to this. Firstly, the arrangement of the fascial elements is more complex in the digit than in the palm, making surgery more demanding and the dissection more extensive. Secondly, the soft tissues supporting the PIPJ also contribute to an intrinsic flexion contracture of the joint, which may persist even when all current disease has been eradicated.
Pathological anatomy
In Dupuytren’s Disease the pathological anatomy is determined by well-defined anatomical pathways with disease involvement of the normal fascial structures present in the digit. Several types of cord may be formed, depending upon which of those fascial structures become involved. Of these, the spiral, central and lateral cords can contribute to the flexion contracture of the PIPJ. One or all three types of cord may be present in a digit. The spiral cord is formed from a confluence of the longitudinal pretendinous fibres in the palm, the spiral band, the lateral digital sheet and Grayson’s ligament, through which it attaches to the middle phalanx. The spiral cord is a major contributor to the PIPJ flexion contracture. It also presents a major surgical hazard as it causes displacement of the neurovascular bundle, putting it at risk of injury during dissection. This is more likely if the PIPJ is severely flexed [3]. In addition, it is now known that Cleland’s ligaments, which are dorsal to the neurovascular bundles, become involved with the disease, having previously been thought not to. Involvement of these structures may also contribute to the PIPJ contracture [4].
Other structures in the digit may contribute to the PIPJ flexion contracture resulting in persisting flexion contracture, even when the contribution by the diseased fascia has been dealt with.
The skin over the palmar surface of the digit is frequently inadequate where there is a significant PIPJ flexion contracture. Although there is no skin loss in Dupuytren’s Disease, the shortening of the diseased fascia allows the skin, which has viscoelastic properties, to shorten also. Management of the skin is a major part of the treatment of Dupuytren’s Disease and many ways have been described to deal with this. These can broadly be classified into two.
Firstly, “redistribution” techniques, which employ those same viscoelastic properties to effectively “redistribute” the skin. These include skin lengthening incisions and open palm techniques. Secondly, the skin can be excised and replaced, usually with a full thickness skin graft. These techniques are dealt with in more detail elsewhere.
When a flexion contracture of the PIPJ has been present for a significant period of time, the volar plate, flexor tendon sheath and collateral ligaments may become shortened, contracted and adherent and contribute to the flexion contracture [5]. This is in contrast to the MCPJ where this is generally not the case.
Historically, various methods have been advocated to deal with this problem, some of which may now not be currently acceptable. These include resection of the PIPJ [6], division of the flexor tendons [7], excision of the proximal phalangeal head [8] and resection of part of the middle phalanx [9]. In the early 20th century, amputation [10] was the preferred option. In 1973, Moberg [11] suggested three ways of avoiding amputation for advanced contractures; PIPJ arthrodesis, dorsal wedge osteotomy of the proximal phalanx and PIPJ arthroplasty. This was reiterated by Tonkin et al. in 1985 [12].
In 1970, Curtis [5] described excision of a portion of the volar plate on either side of the flexor mechanism, occasionally excising the entire volar plate, with postoperative splintage and therapy. In 1979, Watson et al. [13] described a contracture band passing from the proximal edge of the volar plate to the neck of the proximal phalanx. They called this the “check rein ligament” and recommended excision. Eaton had described a similar structure in 1971 [14], although this was in the context of joint injuries.
In 1990, McGrouther [2] noted that the prognosis was poor when surgical PIPJ release was necessary. In a prospective study, Beyermann et al. [15] found no statistically significant difference in the outcome between patients with severe contractures undergoing capsuloligamentous release and those that did not.
Ritchie et al. [16] reported consistently good results with few complications using sequential PIPJ release for severe Dupuytren’s Disease of 11 little fingers. Breed and Smith [17], however, reported that for residual flexion deformity at the PIP joint level after digital fasciectomy, gentle passive manipulation alone gives better results with fewer complications than more aggressive surgical intervention.
Several authors have reported encouraging results when using collagenase to correct PIPJ contractures, when this is injected either into a cord at the MCPJ or at the PIPJ [18], [19]. Although the tissues affected by Dupuytren’s Disease are released with what is effectively a chemical fasciotomy through injection of collagenase, the other tissues that may be contributing to the PIPJ flexion contracture are not addressed, except through manipulation. These encouraging results may reinforce the evidence that a manipulation of the PIPJ is superior to surgical release of the joint.
However, the recurrence of contracture after collagenase injection at three years is reported as higher for the PIPJ (56%) than for the MCPJ (27%) [20].
Initial fears regarding distortion of the anatomy by collagenase injection and affecting subsequent surgery have not been realized [21].
Postoperative
In order to maintain correction and avoid complications, the recommendation of a careful postoperative programme of hand therapy, with judicious splintage is universal [22], [23] [24]. Active mobilization can begin at 3 days but splintage at night may need to continue for 6 months or longer [22]. If a skin graft or open palm technique has been used, initial immobilization may need to continue for longer, prior to active mobilization; McGrouther [23] recommended 10 days. After collagenase injection, Hurst [24] recommends night splintage in extension for three months. After PIPJ correction has been made, the splint will need frequent adjustment and if correction has been incomplete, dynamic splintage is of value.
Beard and Trail [25] advocated the use of a dynamic external fixation after surgery, which improved the initial correction in 17 out of 18 fingers. Only five patients maintained this further correction, however.
Author’s preferred technique
For significant PIPJ contractures a regional fasciectomy is preferred. Under regional or general anaesthesia the arm is exsanguinated with an Esmarch bandage, which is left in place for 45 seconds before the pneumatic tourniquet is inflated. 3.5 loupe magnification is used.
A midline straight incision is made over the digit, angled at the distal interphalangeal joint and in the palm. The skin here does not readily transpose and therefore does not lend itself to Z-plasties.
A midline incision over the digit is preferred as this provides better access to both sides of the digit and ability to perform Z-plasties as necessary. Sharp dissection is used, using fresh blades, which are changed frequently. Scissors are not employed due to risk to the neurovascular structures.
After excision of disease in the palm, the digital disease is carefully split in the midline from distal to the PIPJ to the base of the digit. As long as the midline is not crossed the neurovascular structures will be safe, as these do not cross the true midline. The presence of Pacinian (Lamellar) corpuscles, which are easily visible in the perineural fat, will alert the surgeon to the proximity of a digital nerve.
Once the disease has been split longitudinally, each half of the disease can be dealt with separately and the digital neurovascular bundles can be identified on each side, before resection of the disease. The surgeon sits at the hand table facing the disease being dissected, changing sides as necessary. If the bundles are identified proximal and distal to the disease on each side, these structures can be followed using sharp dissection to divide the disease, which lies over them. Once the bundles have been identified throughout the length of the dissection the disease is excised. A specific dorsal retro-vascular dissection is made just distal to the PIPJ to identify the Cleland’s ligaments on each side. These, which are often involved with disease and may contribute to the contracture, are excised (Figure 1).
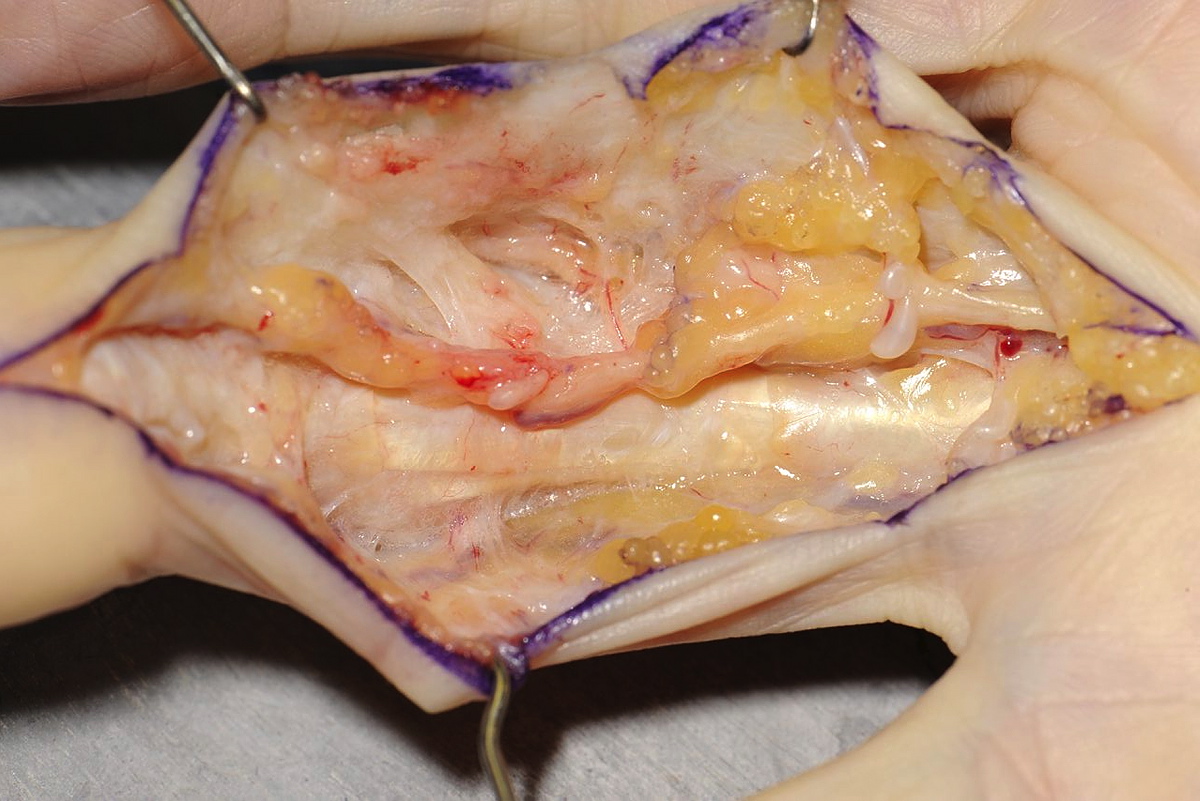
If the flexor sheath is contracted then this is released with a transverse incision between the A2 and A3 pulleys. If there is a residual flexion deformity of the joint then this is corrected with a very gentle passive stretch. Care is taken not to hyperextend the joint. Surgical soft tissue release of the joint is not performed.
The skin is closed with several Z-plasties. If the contracture is recurrent or particularly severe then a segment of skin is excised and cover is obtained with a full thickness skin graft taken from the medial side of the elbow. The graft extends to the mid-axial point of the digit so as to form a firebreak across the full width of the glabrous skin. A tie-over suture is not used but the graft is perforated with multiple slits to prevent accumulation of haematoma and, after release of the tourniquet, direct pressure is applied through the dressings by the recovery staff for 15 minutes.
Broad spectrum antibiotics are prescribed for 5 days. A palmar plaster splint is used for rest and comfort until the third post-operative day when the patient is seen by the therapists.
A resting splint is fabricated from thermoplastic material, to be worn at night and intermittently during the day, as necessary, and the patient is instructed on exercises to be performed regularly. If a skin graft has been used, the digit is splinted continuously until the sutures are removed at 12 days.
Conclusions
Correction of the severe PIPJ contracture in Dupuytren’s Disease remains a significant challenge.
The literature regarding soft tissue release of the joint is conflicting, but a thorough excision of the disease followed by gentle passive stretching of the joint yields results as good as more extensive surgery. The use of full thickness skin grafting plays a role not only for skin cover but also in prevention of recurrence. Collagenase has a role to play in the correction of PIPJ contracture, which will become more defined over the next few years. Postoperative hand therapy with judicious splintage is mandatory.
References
[1] Dupuytren G. De la rétraction des doigts par suite d’une affection de l’aponéurose palmaire – description de la maladie – operation chirurgicale qui convient dans ce cas. Compte rendu de la clinique chirurgicale de l’Hotel Dieu par MM les docteurs Alexandre Paillard et Marx. J universel et hebdomadaire de médecine et de chirurgie pratiques et des institutions médicales. 1831;5:349-365.[2] McGrouther DA. An overview of operative treatment. In: McFarlane RM, McGrouther DA, Flint MH, editors. Dupuytren’s Disease: Biology and Treatment. Edinburgh: Churchill Livingstone; 1990. p. 309-10.
[3] Umlas ME, Bischoff RJ, Gelberman RH. Predictors of neurovascular displacement in hands with Dupuytren's contracture. J Hand Surg Br. 1994 Oct;19(5):664-6.
[4] Shewring DJ, Rethnam U. Cleland's ligaments and Dupuytren's disease. J Hand Surg Eur Vol. 2014 Jun;39(5):477-81. DOI: 10.1177/1753193413510440.
[5] Curtis RM. Capsulectomy of the interphalangeal joints of the fingers. J Bone Joint Surg Am. 1954 Dec;36-A(6):1219-32.
[6] Nélaton; Société et Chirurgerie. Séance du 25 juin. Bulletin et Mémoires de la Sociétés des Chirurgiens de Paris. 1908;34:803.
[7] Kosininski K. Operativ – Orthopadische Behandlung der Dupuytren’schen Kontraktur. Chirurgerie Narzadow Ruchu. 1940;11:283.
[9] Hutchinson J. Dupuytren’s contracture of the palmar fascia. Lancet. 1917;95:285.
[10] Eckstein H. Phalangenresektion zur Beseitigung von Fingerkontraktur. Zentralbl Chir. 1922;49:547.
[11] Hakstian RW. Long-term results of extensive fasciectomy. Br J Plast Surg. 1966 Apr;19(2):140-9.
[12] Moberg E. Three useful ways to avoid amputation in advanced Dupuytren's contracture. Orthop Clin North Am. 1973 Oct;4(4):1001-5.
[13] Tonkin MA, Burke FD, Varian JP. The proximal interphalangeal joint in Dupuytren's disease. J Hand Surg Br. 1985 Oct;10(3):358-64.
[14] Watson HK, Light TR, Johnson TR. Checkrein resection for flexion contracture of the middle joint. J Hand Surg Am. 1979 Jan;4(1):67-71.
[15] Eaton RG. Joint injuries of the hand. Springfield: Thomas; 1971.
[16] Beyermann K, Prommersberger KJ, Jacobs C, Lanz UB. Severe contracture of the proximal interphalangeal joint in Dupuytren's disease: does capsuloligamentous release improve outcome? J Hand Surg Br. 2004 Jun;29(3):240-3.
[17] Ritchie JF, Venu KM, Pillai K, Yanni DH. Proximal interphalangeal joint release in Dupuytren's disease of the little finger. J Hand Surg Br. 2004 Feb;29(1):15-7.
[18] Breed CM, Smith PJ. A comparison of methods of treatment of pip joint contractures in Dupuytren's disease. J Hand Surg Br. 1996 Apr;21(2):246-51.
[19] Manning CJ, Delaney R, Hayton MJ. Efficacy and tolerability of Day 2 manipulation and local anaesthesia after collagenase injection in patients with Dupuytren's contracture. J Hand Surg Eur Vol. 2014 Jun;39(5):466-71. DOI: 10.1177/1753193413490899
[20] Warwick D, Arner M, Pajardi G, Reichert B, Szabo Z, Masmejean EH, Fores J, Chapman DS, Gerber RA, Huard F, Seghouani A, Szczypa PP; POINT X Investigators. Collagenase clostridium histolyticum in patients with Dupuytren's contracture: results from POINT X, an open-label study of clinical and patient-reported outcomes. J Hand Surg Eur Vol. 2015 Feb;40(2):124-32. DOI: 10.1177/1753193413519926
[21] Peimer CA, Blazar P, Coleman S, Kaplan FT, Smith T, Tursi JP, Cohen B, Kaufman GJ, Lindau T. Dupuytren contracture recurrence following treatment with collagenase clostridium histolyticum (CORDLESS study): 3-year data. J Hand Surg Am. 2013 Jan;38(1):12-22. DOI: 10.1016/j.jhsa.2012.09.028
[22] Hay DC, Louie DL, Earp BE, Kaplan FT, Akelman E, Blazar PE. Surgical findings in the treatment of Dupuytren's disease after initial treatment with clostridial collagenase (Xiaflex). J Hand Surg Eur Vol. 2014 Jun;39(5):463-5. DOI: 10.1177/1753193413488305
[23] Mackin EJ, Byron PM. Postoperative management. In: McFarlane RM, McGrouther DA, Flint MH, editors. Dupuytren’s Disease: Biology and Treatment. Edinburgh: Churchill Livingstone; 1990. p. 368-9.
[24] McGrouther DA. Dupuytren’s Contracture. In: Green DP, Hotchkiss RS, Pederson WC, editors. Green’s Operative Hand Surgery. 4th ed. Edinburgh: Churchill Livingstone; 1998. p. 582-5.
[25] Hurst L. Dupuytren’s Contracture. In: Green DP, Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH, editors. Green’s Operative Hand Surgery. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2011. p. 153-5.



