Surgical treatment for Dupuytren's Disease: open fasciotomy and fasciectomy
1 Hornsby Hand Centre, Hornsby, Australia
Introduction
The traditional role of surgery in the treatment of Dupuytren’s Disease is being challenged by less invasive treatment options, specifically percutaneous needle fasciotomy and, more recently, collagenase injection. Nonetheless, surgery maintains an important place in the management of this condition. For many surgeons and in many situations, for example multiple digit involvement, recurrent disease, severe contractures and where collagenase is unavailable or unaffordable, surgery remains the primary treatment.
Open fasciotomy
The simplest surgical treatment is open fasciotomy. As the name implies, this procedure involves division of a Dupuytren’s cord through a skin incision, that is, under direct vision. Thus no disease is actually excised. Open fasciotomy has some advantages over percutaneous needle fasciotomy in that since the procedure is done under direct vision, the surgeon can ensure that the division is complete and the risk of injury to the digital arteries and nerves and the flexor tendons is reduced. Open fasciotomy is usually performed in the palm but it can be performed more distally or at multiple levels in the same digit. While a full correction of an isolated metacarpophalangeal joint (MCPJ) contracture could well be expected using this technique, given the limited nature of the procedure complete correction of more severe or complex contractures is unlikely, especially for the proximal interphalangeal joint (PIPJ) where the disease is often less well defined. Even if a full correction is not achieved open fasciotomy may be able to improve a contracture to some degree so as to permit some improvement in hand function, hygiene and cosmesis. The limitations of the technique generally restrict its use to patients or surgeons wanting minimal surgery, patients with severe contractures requiring staged surgery and palliative surgery in patients who are unfit for a longer operation.
Fasciectomy
Fasciectomy involves removing diseased fascia from the target ray or rays. Fasciectomy can segmental, partial, or total, or be part of a dermofasciectomy procedure.
Segmental fasciectomy involve removing one or more short segments of the Dupuytren’s cord/s. It may be done at one level, for example, in the palm, or at multiple levels, but the disease in between is left in situ. While segmental fasciectomy is the treatment of choice for some surgeons it may be especially indicated in certain situations e.g. stage one of a two stage procedure in a patient with a severe contracture, or a limited procedure in an unfit patient.
Partial (also called limited, subtotal or selective) fasciectomy aims to remove the diseased tissue while leaving behind the normal fascia. The term ‘partial’ and related terms cover the whole range of degrees of excision between ‘segmental’ and ‘total’. This creates some difficulty in analyzing and comparing results between different studies. Ideally, most, if not all, of the diseased tissue would be removed, but at a minimum at least that tissue responsible for the contracture needs to be excised. Disease located proximally in the palm can be left to involute.
Total (or radical) fasciectomy aims to remove all the fascia, both diseased and normal, and therefore involves a more extensive dissection and exposure.
Dermofasciectomy involves excision of skin along with the diseased fascia. As it is a more radical approach, it is usually reserved for situations where there is longitudinal skin shortage, where there is recurrent disease with dense skin involvement, where skin is devitalized during surgery or as a primary procedure in young patients with a strong Dupuytren’s diathesis [1], [2].
Indications for surgery
The indications for surgery for Dupuytren’s Disease vary between surgeons, but are usually based on the degree of fixed flexion contracture, especially at the PIPJ. A flexion contracture at the MCPJ is more easily corrected and the correction gained is more easily maintained, because the collateral ligaments in this joint are most stretched when the joint is flexed. Therefore in extending the joint from the contracted position the ligaments relax and do not impede correction of the contracture. A contracture at the PIPJ however, is more difficult to correct and maintain, because the collateral ligaments and the volar plate become contracted when the joint is flexed, so extending this joint tightens these structures further, and they can contribute to a residual or recurrent contracture. For these reasons, many surgeons will recommend surgery for the PIPJ at a lesser degree of contracture than the MCPJ. Some surgeons even recommend any degree of PIPJ contracture warrants surgery. The problem with this approach is that if one operates on a PIPJ that has a minor contracture there is a significant risk that, with post-operative swelling and scarring, the patient may not be any better off, or may even be worse off following the surgery. Conversely, with the MCPJ, even a moderate contracture can be safely observed if it is not causing any functional difficulty for the patient, because MCPJ contractures can be readily corrected later. The most common cut-off for recommending surgery is forty degrees of contracture at the MCPJ and twenty degrees of contracture at the PIPJ [2]. The author prefers a more conservative approach, that being: for MCPJ’s, the indication for surgery is a degree of contracture that is causing sufficient functional problems for the patient (for example washing one’s face, shaking hands with others, putting one’s hand in one’s pocket) and for PIPJ’s the indication for surgery is a thirty to forty degree contracture. Of course many patients present for surgery with much more advanced contractures and the worse the pre-operative contracture is, the more difficult it is to achieve full extension intra-operatively and maintain it post-operatively.
Dupuytren’s Disease in the thumb is commonly seen but does not commonly need surgery. Two main cords have been identified affecting the thumb: one along the radial border of the thumb and the other across the first web [3]. Surgery is indicated in the presence of a significant web contracture, or less commonly, a significant flexion contracture of the thumb MCPJ.
Dupuytren’s Disease may also need to be excised if it is overlying the A1 pulley of a trigger finger that needs surgery, even if the Dupuytren’s is not causing a contracture, and also, on rare occasions, when it presents as an isolated nodule and histological confirmation of the diagnosis is desired to rule out a malignancy.
Pre-operative planning
The surgery can be performed through a number of approaches, and there are a number of options available in repair and management of the surgical wound. The disease pattern needs to be carefully assessed and decisions need to be made with regards to the extent of the surgery. For example, there may be a predominant ray affected, but it may be possible to resect disease from the palm of an adjacent ray or a natatory cord by raising a flap or a minor extension of the incision. Two or more rays may need addressing and the incisions in these cases will need to accommodate this and be carefully planned in order to avoid narrow skin bridges and devitalized flaps. Note needs to be made of any macerated areas that should be cleaned and dried out in the days before the surgery. Also any calloused skin can be softened with moisturizer in the weeks before the surgery to make skin flap handling easier. Deep pits need to be noted to avoid buttonholing the flaps in those areas. An area of fat between the skin and the cord at the base of the finger needs to be noted because it can be indicative of a spiral cord. For severe contractures a full thickness skin graft (FTSG) may be required and the donor site (e.g. transversely in the cubital fossa) should be assessed for integrity and preferably hairlessness. The strength of the extensor tendon mechanism and the range of flexion should also be noted. In cases of severe and longstanding contractures, and for patients with known osteoarthritis, a pre-operative x-ray is useful in documenting the status of the joint itself, and helping to anticipate recalcitrant joint contractures.
Fasciotomy is usually done through a simple transverse skin incision at the desired level. The planning of the skin incisions for fasciectomy however needs to be done with particular care, with attention paid to the pattern and severity of the disease and the options for wound closure/management. Here are several possible approaches:
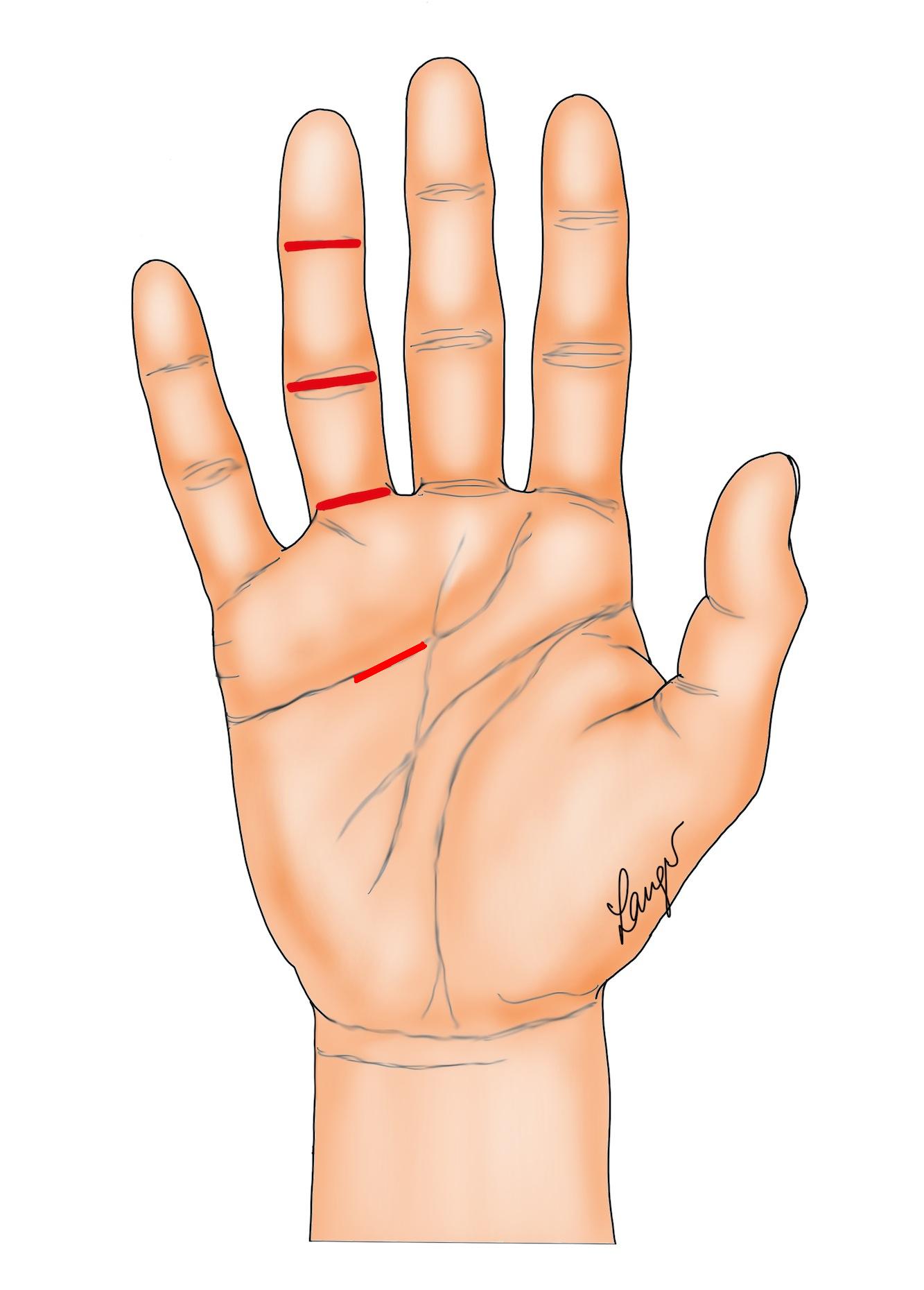
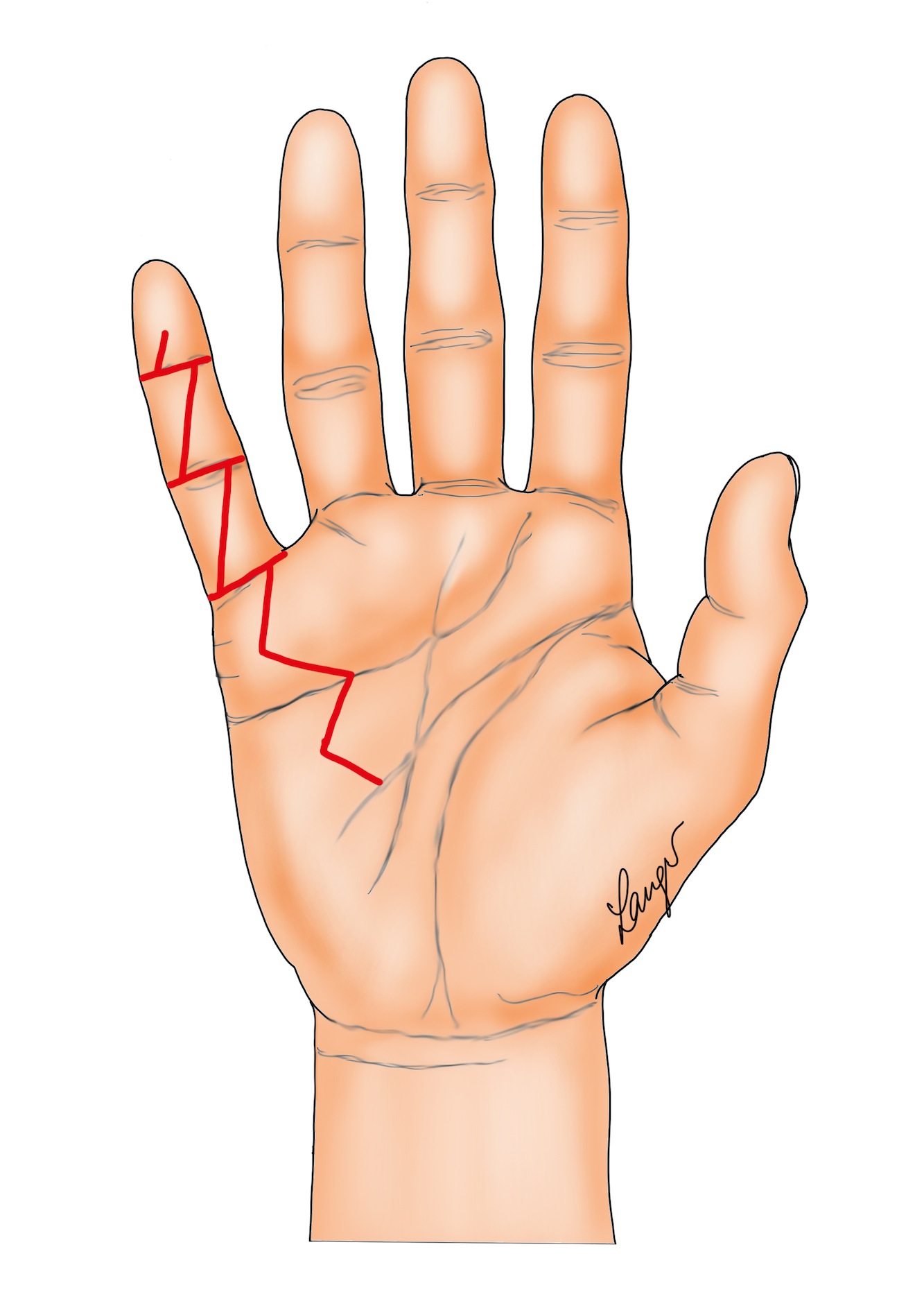
1. Segmental excision through multiple transverse wounds in the joint creases, with the wounds closed primarily, left open or skin grafted (Figure 1) [4].
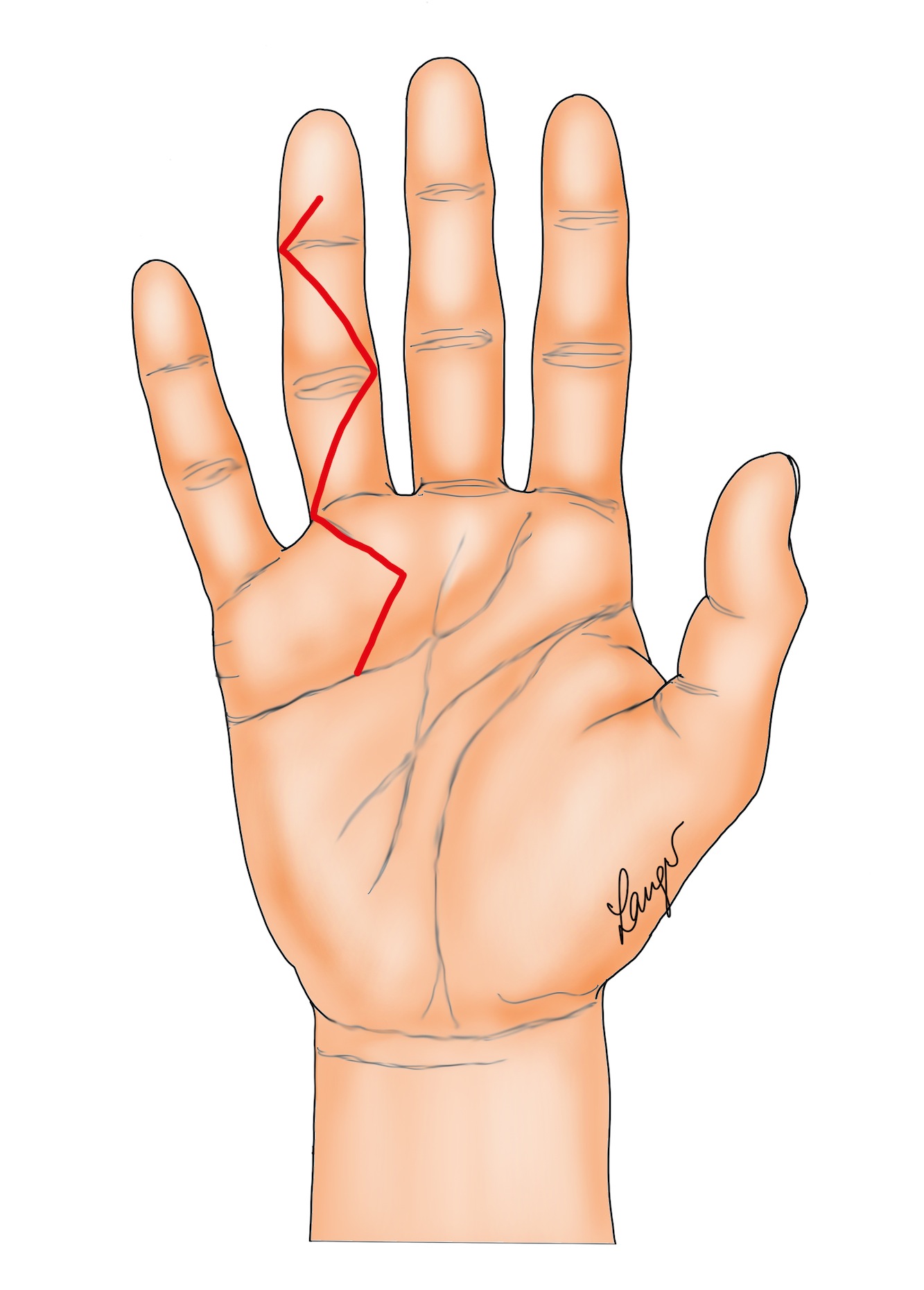
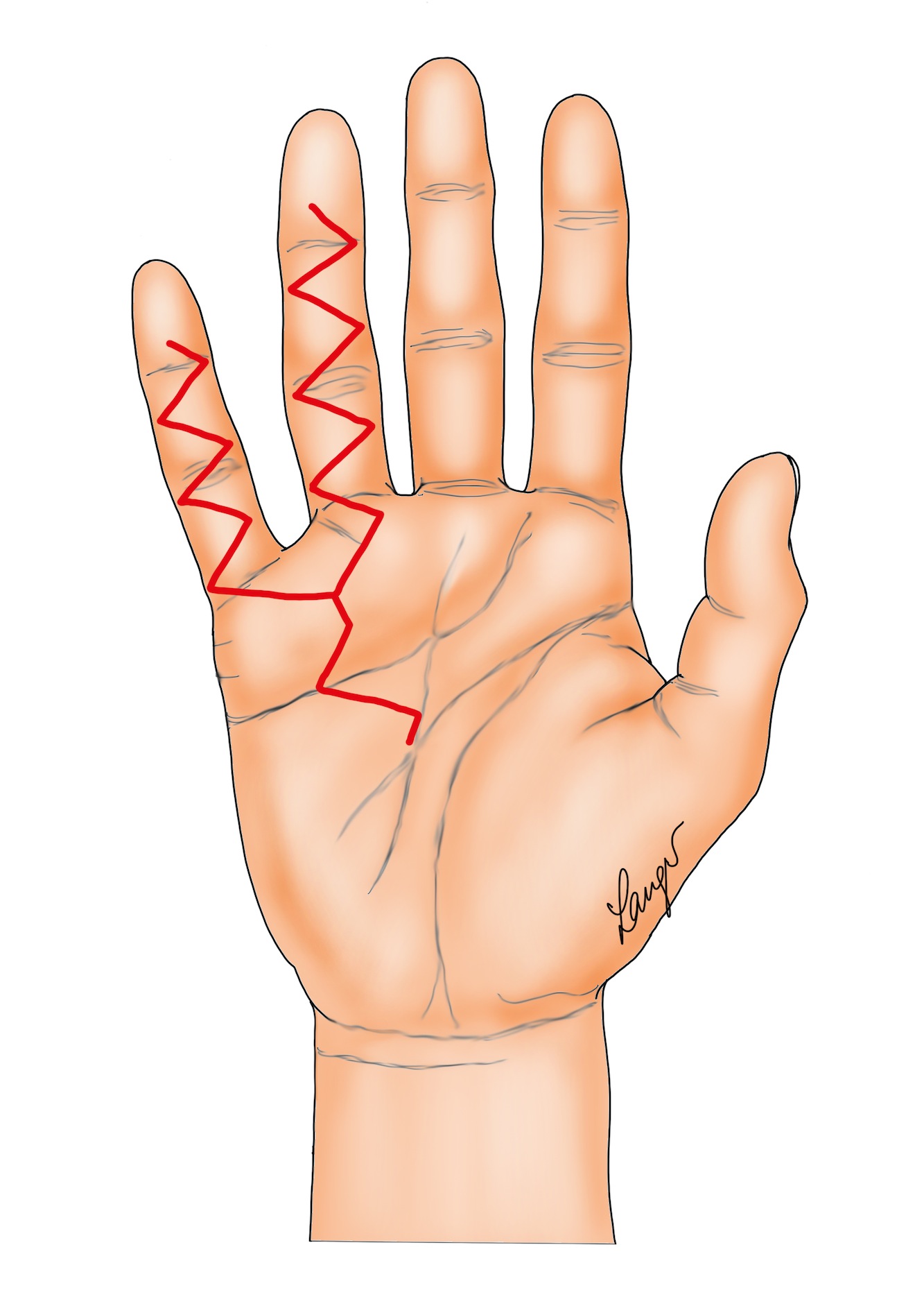
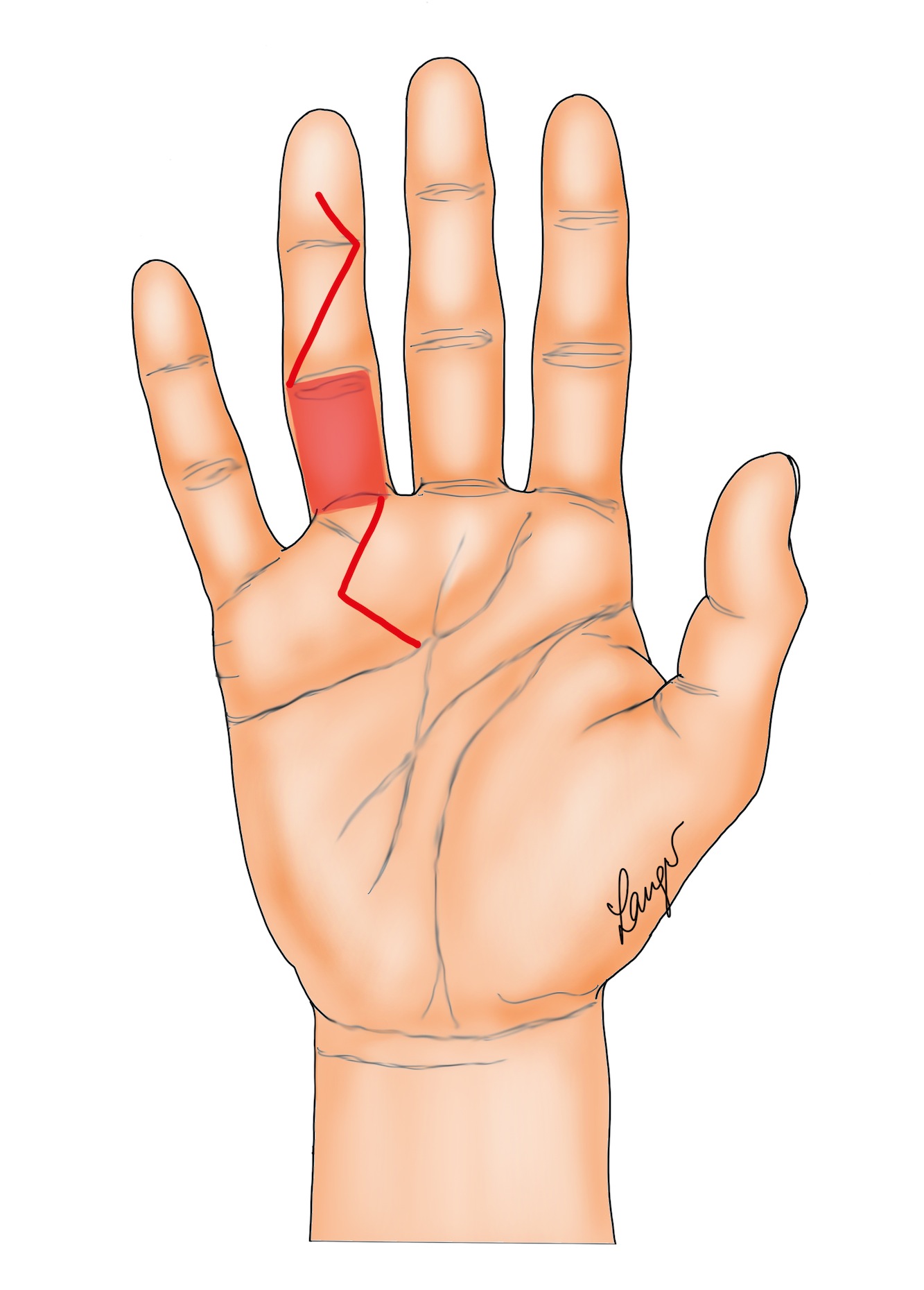
2. Formal Bruner incisions with direct closure (Figure 2) [5] or the variation contributed by our illustrator Martin Langer (Figure 3).
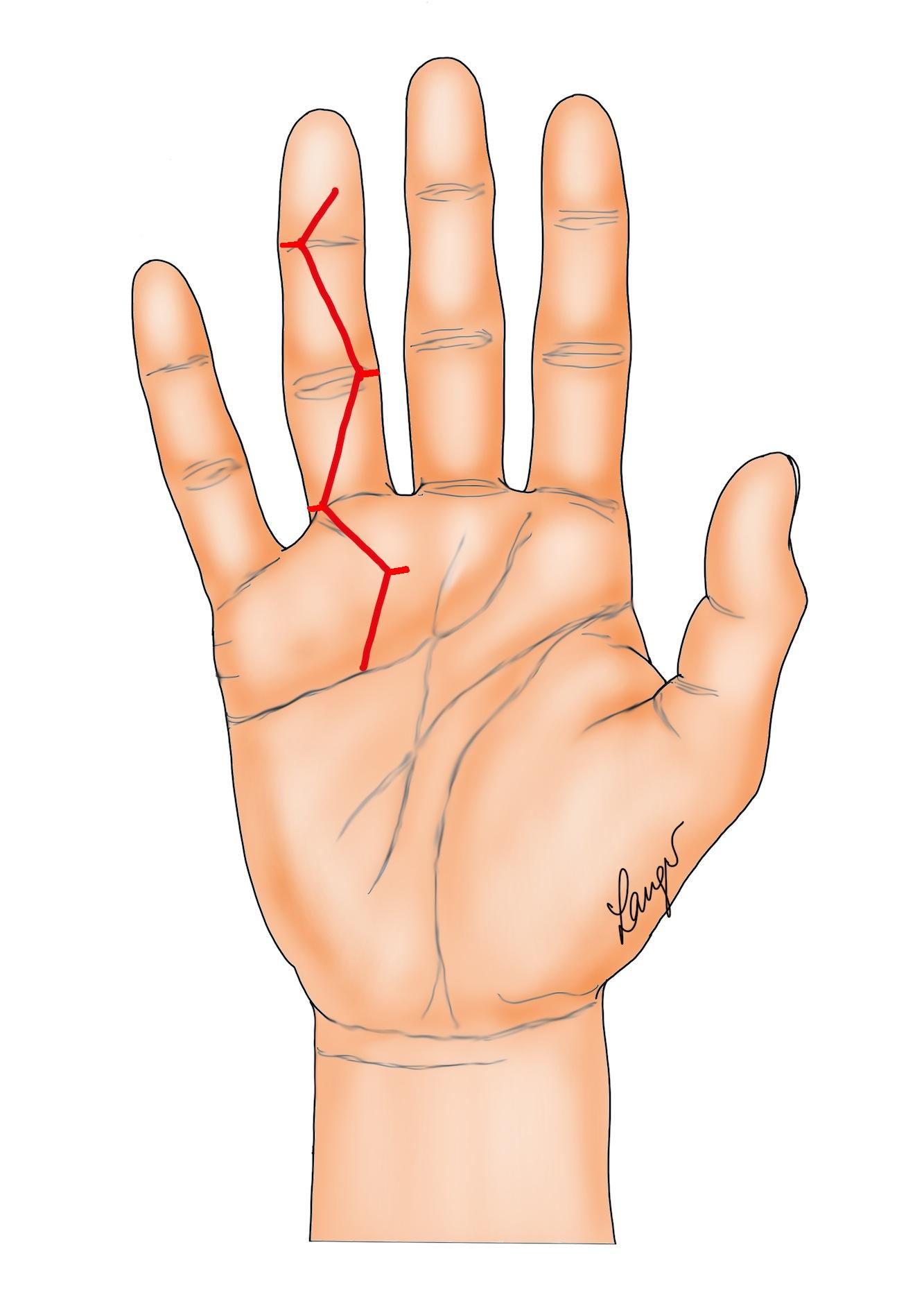
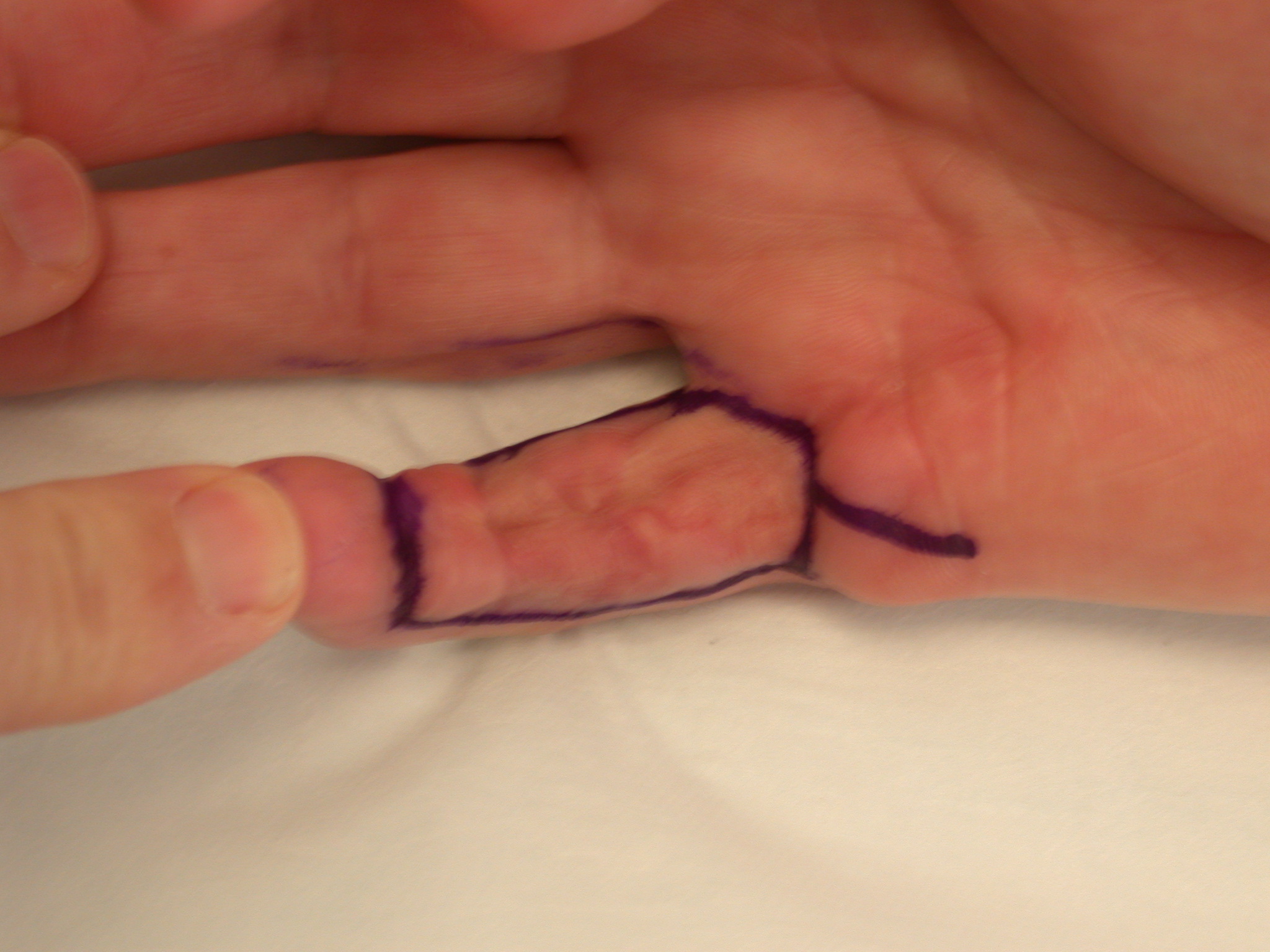
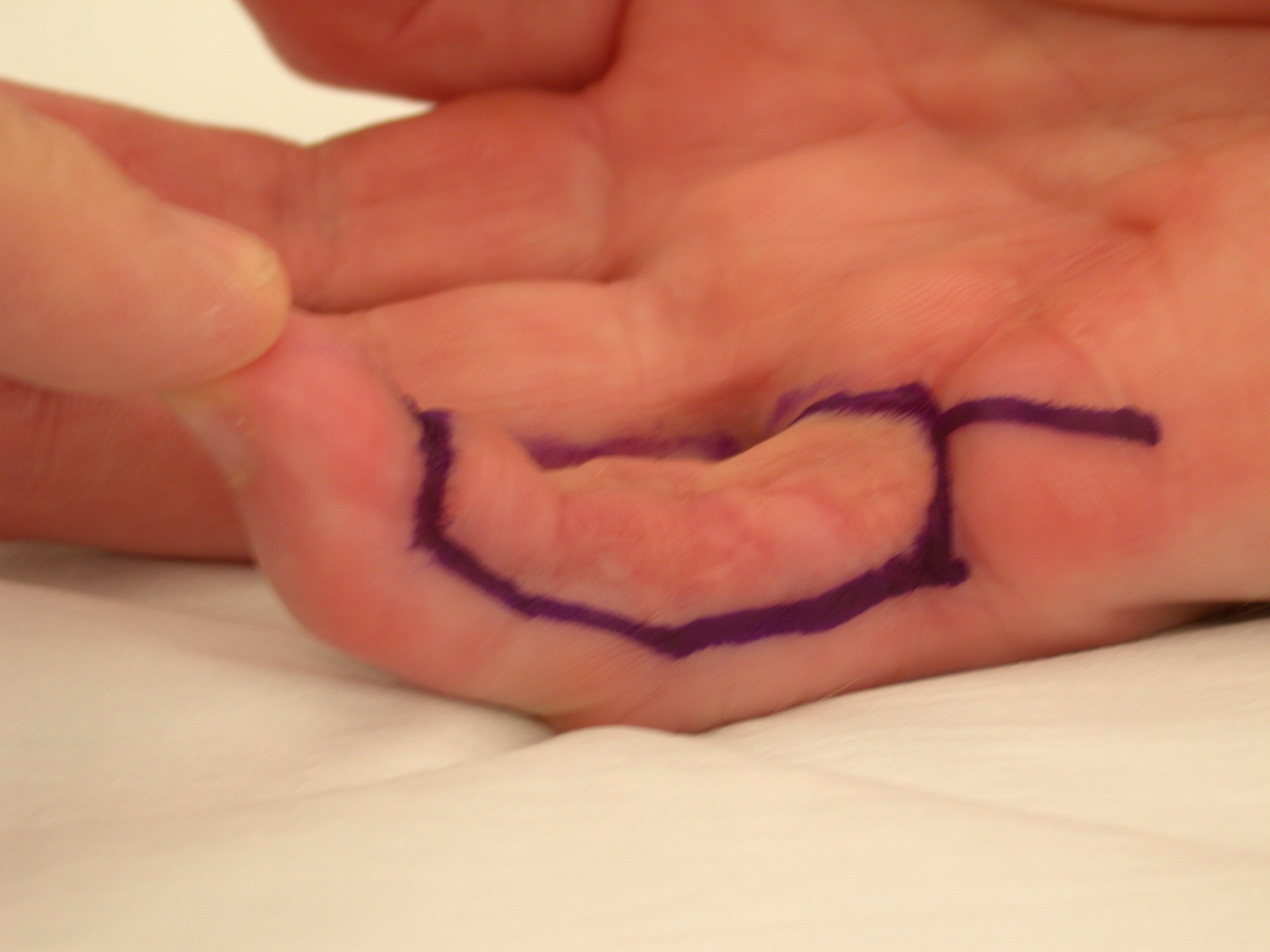
3. “Lazy” (i.e. not extending to the mid-lateral lines) Bruner incision with multiple Y-V advancement flaps (Figure 4) [6] or Palmen’s original version of this (Figure 5) [7].
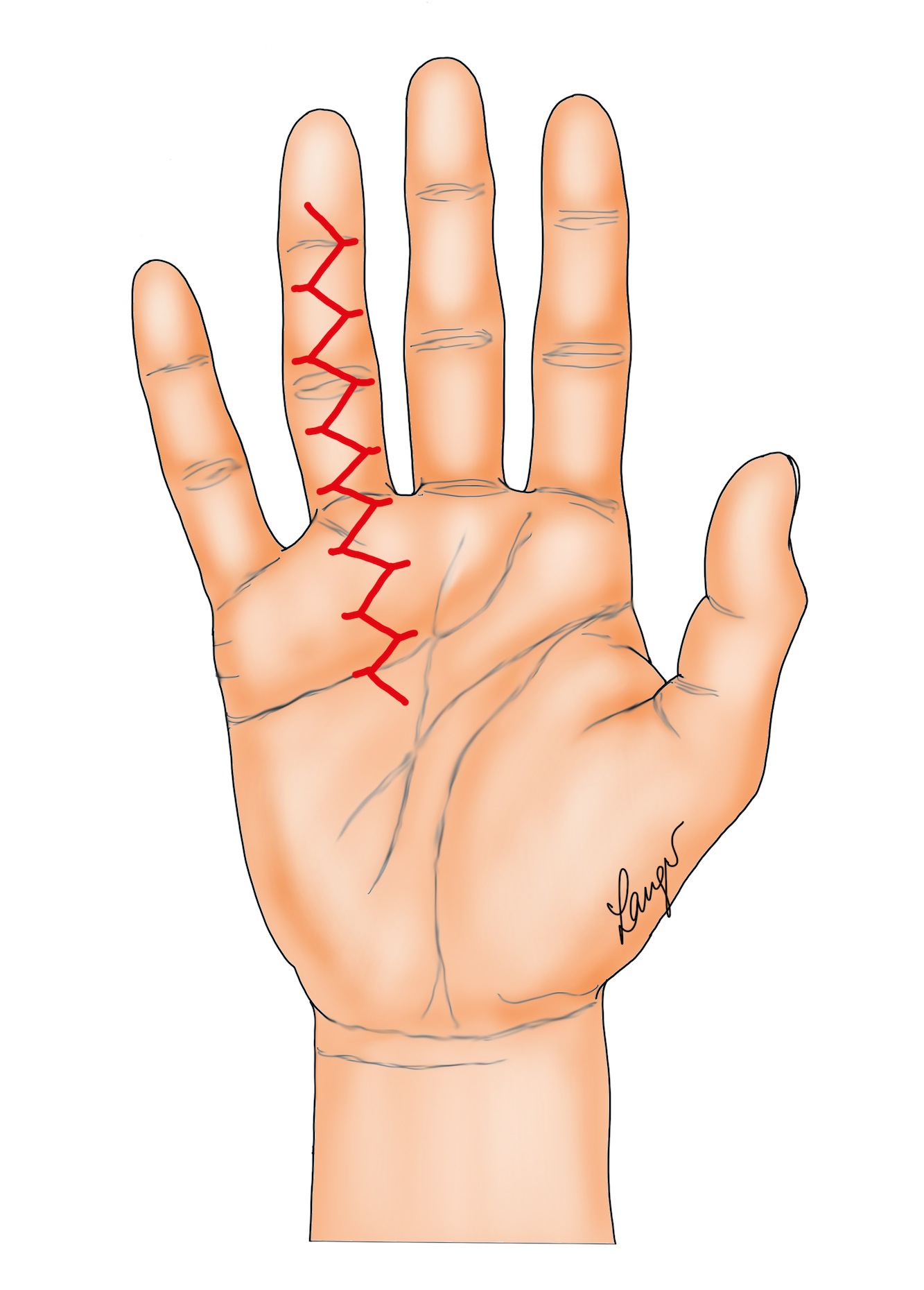
4. Straight longitudinal incision with Z-plasty closure. Note the Z-plasties can be designed so that the transverse limbs of the incision when closed lie over the joint creases as illustrated (Figure 6a) or half way between the joint creases (Figures 6b and 6c). The latter is easier because of the relatively looser skin in the mid-portion of each segment. Also note Z-plasties in the palm are more difficult to mobilize and advance because the skin is less mobile.
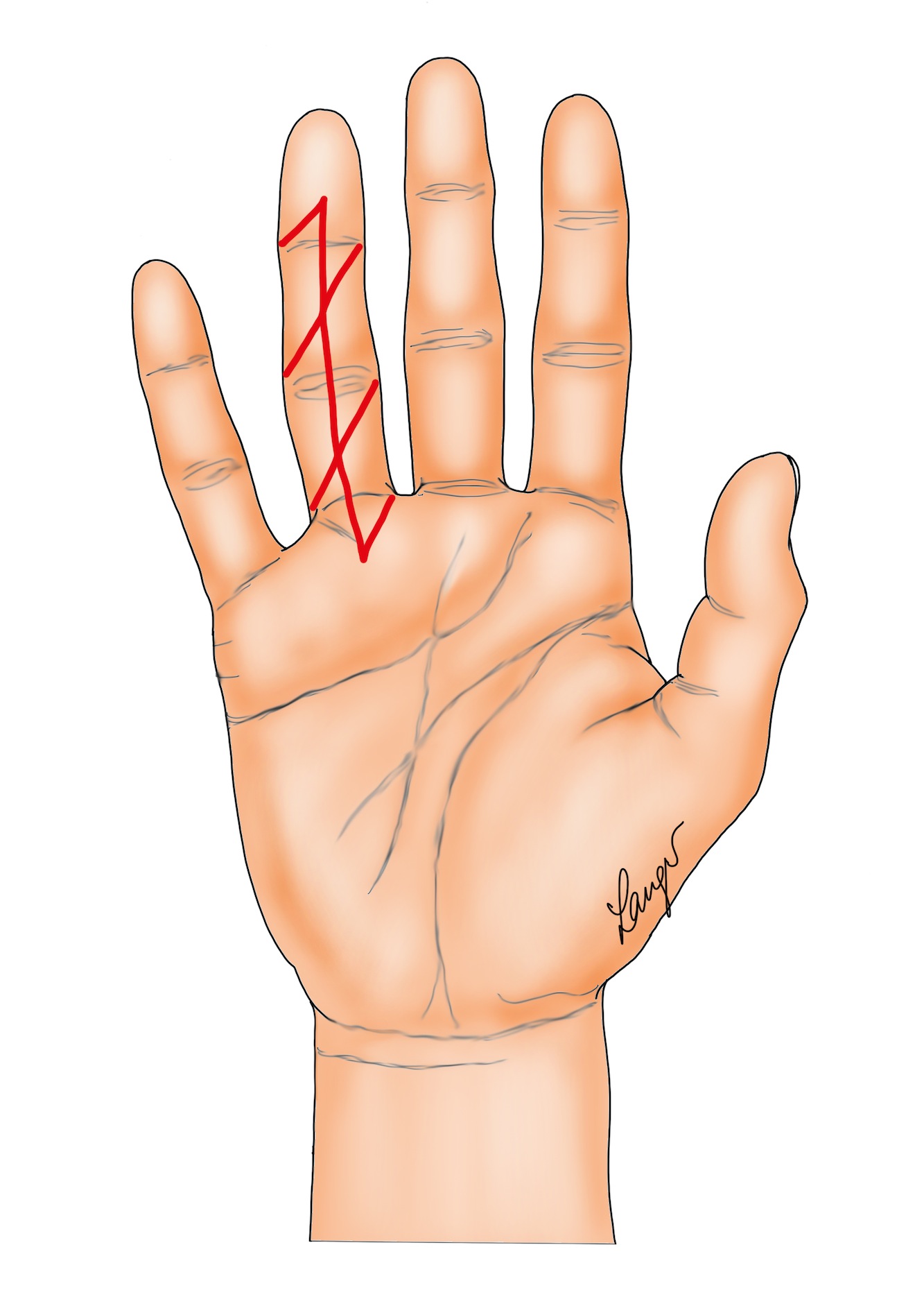
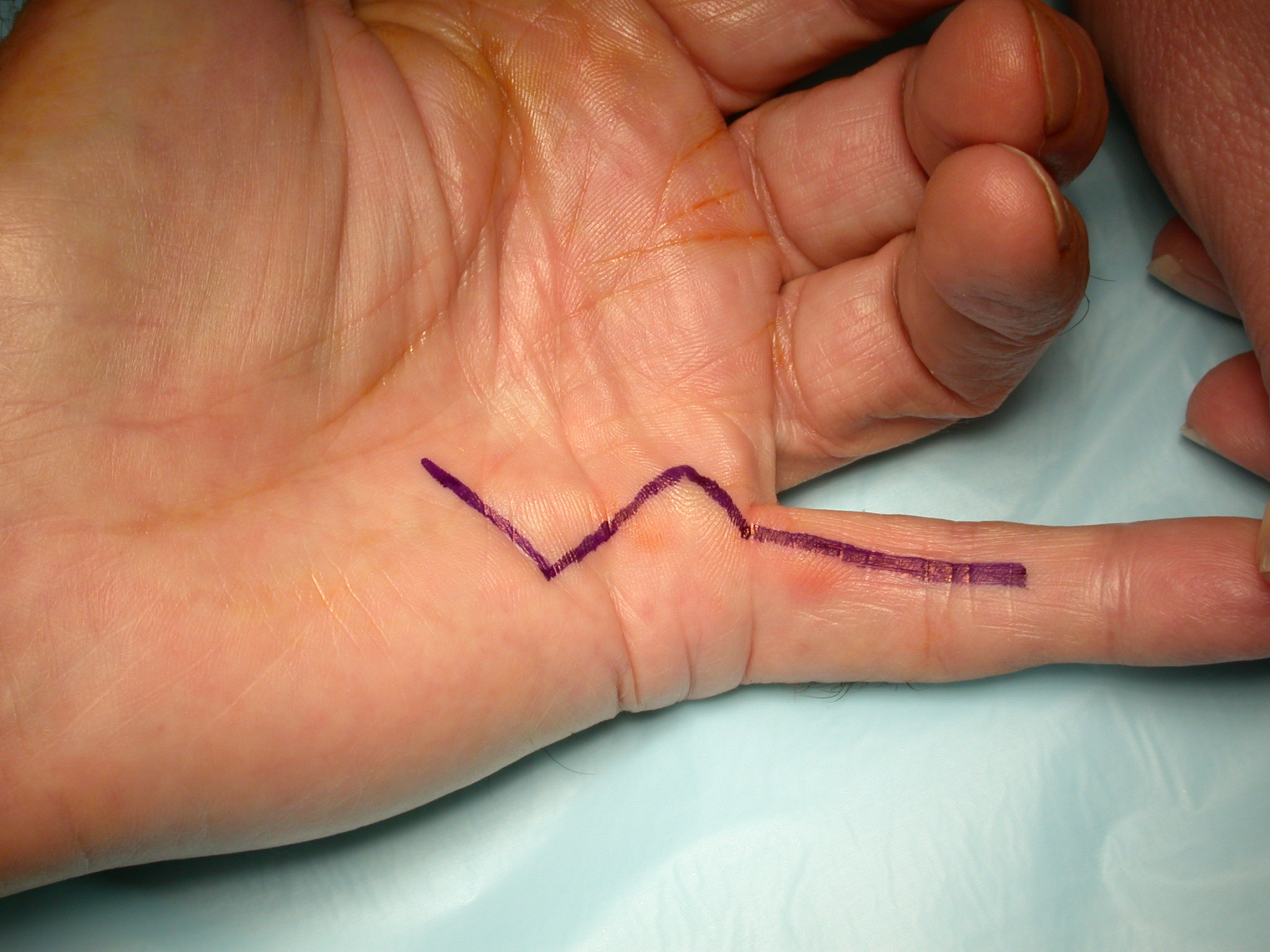
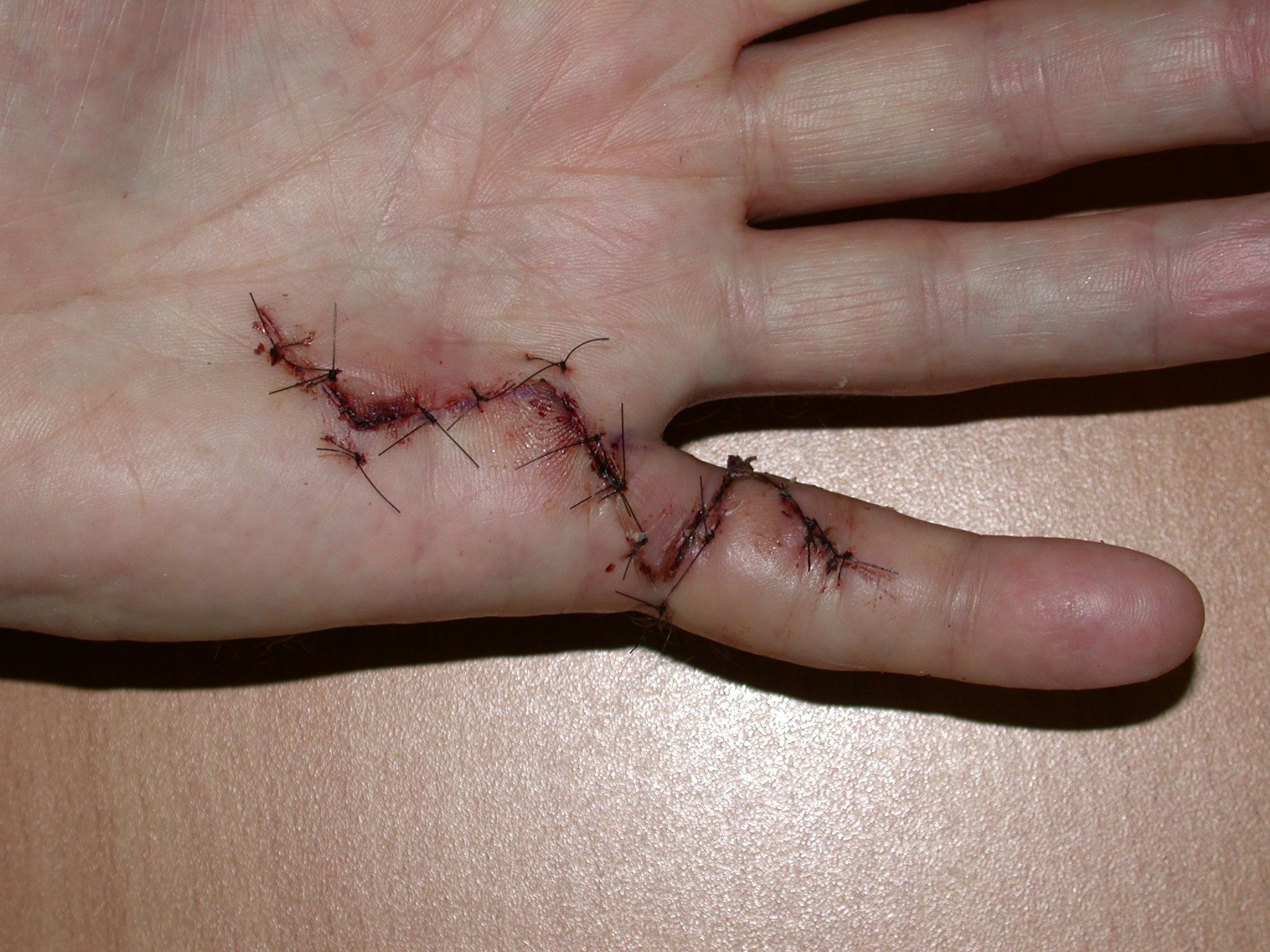
5. “Modified Bruner” incisions which consist of transverse incisions in the joint creases joined by oblique incisions at 45 degrees (Figure 7). These are suitable for direct closure in mild contractures; otherwise multiple Y-V advancement flaps are used, with the transverse sections of the wounds being closed primarily, left open or skin grafted (see details below).
6. Dermofasiectomy, which consists of excision of skin with the diseased fascia, which necessitates skin grafting (Figure 8a-e).
Surgical Technique
Open fasciotomy is often done under local anaesthetic through a transverse incision, although a longitudinal incision has also been recommended to allow easier wound closure [7]. The fasciotomy is usually done at a level where the cord is well defined and superficial, most often in the palm. Local skin flaps are raised and the cord is exposed and simply divided. The wound is closed if possible, otherwise it is left open to heal by secondary intention.
Due to the duration of the procedure fasciectomy is usually done under a general anaesthetic. A tourniquet is always required, and loupe magnification highly recommended. The surgery proceeds conceptually in the following steps, and most of these principles apply regardless of the skin incisions used:
- Skin incisions are planned, marked and made (some examples are shown in figures 1–8).
- Skin flaps are raised. It is desirable not to leave disease on the under surface of the skin flaps, so in areas where the disease is quite superficial, the flaps are raised very thinly, consisting of no more than the skin. In other areas it is possible to leave some fat with the skin to aid skin vascularity. Each skin flap is raised laterally until the dissection is clear of the disease, a boundary which is usually indicated by the presence of fat. This dissection is aided by extension of the transverse incisions to or beyond the mid-lateral lines (if employing the ‘modified Bruner’ approach). This subcutaneous plane is the first of two ‘safe’ planes in terms of the risk of injury to the neuro-vascular bundles.
- The neuro-vascular bundles are dissected out and protected. Particular care is taken in the presence of a spiral, or even double spiral, cord [8].
- The disease is excised, including the obvious pre-tendinous cords but also the less apparent lateral digital and retrovascular cords, and, in the case of the little finger, any abductor digiti minimi cord [9]. In excising the disease one makes use of the second ‘safe’ plane which is between the pre-tendinous cord and the flexor sheath.
Steps 2, 3, and 4 are often done concurrently, depending on the pattern of the disease and the surgeon’s preference. For example, in the case of a severe contracture, it may be necessary to release the palmar disease to allow partial straightening of the digit before the surgery extends into the finger itself. - A gentle manipulation of the contracted joints is attempted. In the event of a persistent fixed flexion deformity, an inspection is made for any residual disease, or any other tight structures prohibiting full joint extension. These might include skin, flexor tendon sheath, collateral ligaments and volar plate. If faced with a significant residual contracture (twenty degrees or more, although some authors recommend thirty degrees as a cut-off [10], [11]) then a formal joint release is performed. This entails an incision in the flexor sheath releasing the A3 pulley, and then serial releases of the medial and lateral walls of the sheath, accessory collateral ligaments, volar plate and collateral ligaments proper from their origin. An attempt at gentle manipulation into full extension is performed after each stage of release. In the presence of significant osteoarthritis of the PIPJ a full correction may not be achievable.
- The tourniquet is deflated, the wound is irrigated and haemostasis achieved, and the finger circulation is assessed. In the event of a white finger with intact digital arteries, a peripheral nerve block, a warm sponge to cover the hand, relaxing the extension of the digit and time are sufficient to restore adequate circulation.
- The wound is assessed for closure options and reconstructed accordingly.
- A long acting local anaesthetic wrist block or local infiltration is performed.
- A dressing and splint (if desired) are applied.
Author’s preferred method
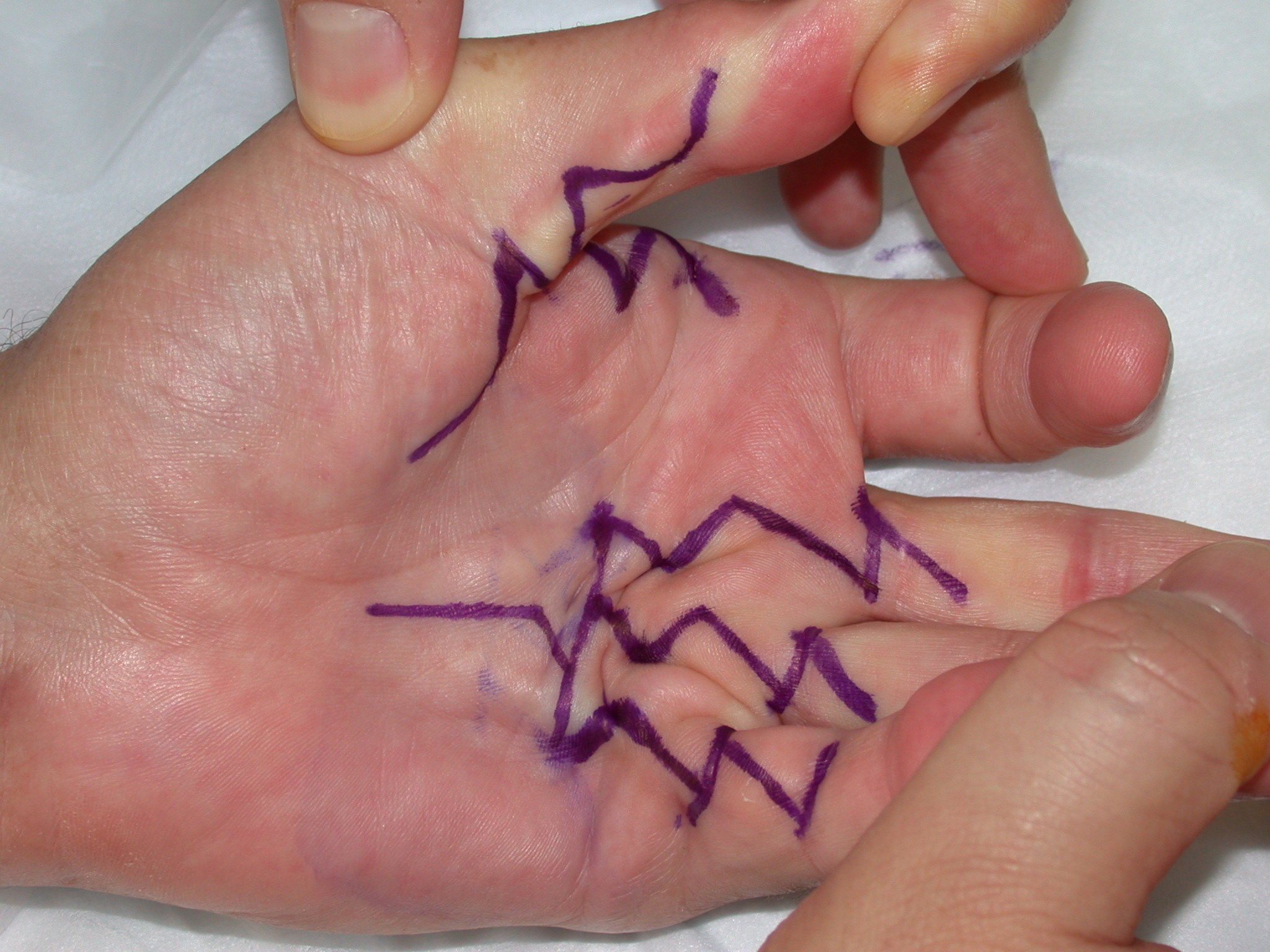
The ‘modified Bruner’ approach is the author’s preferred method (Figure 7) [12]. The approach is based on wide transverse incisions in the skin creases of the finger, joined by oblique incisions at 45 degrees. Note that the oblique incisions do not initially extend to the mid-lateral lines but the transverse incisions do. The incision is extended as far into the palm as required, usually in a zig-zag fashion with either an apex at or a limb along each of the palmar skin creases. The same basic pattern for the incision can be used on any ray and on adjacent rays (Figure 9b–c), regardless of the severity of the contracture. The incision can be placed eccentrically on the digit, being more ulnar or radial if the disease is not central. The incision avoids hypertrophic scarring at the basal finger crease (which is seen with other incisions (Figures 2–6) because the apex of those incision at the basal finger crease does not reach the mid-lateral line, being limited by the web). The flaps are smaller than the original Bruner flaps (Figure 2) [5] and thus easier to raise with less risk of buttonholing and flap ischaemia. The exposure gained is excellent (Figure 10), including to lateral disease which, if not removed, is a cause of residual or early recurrent contracture.
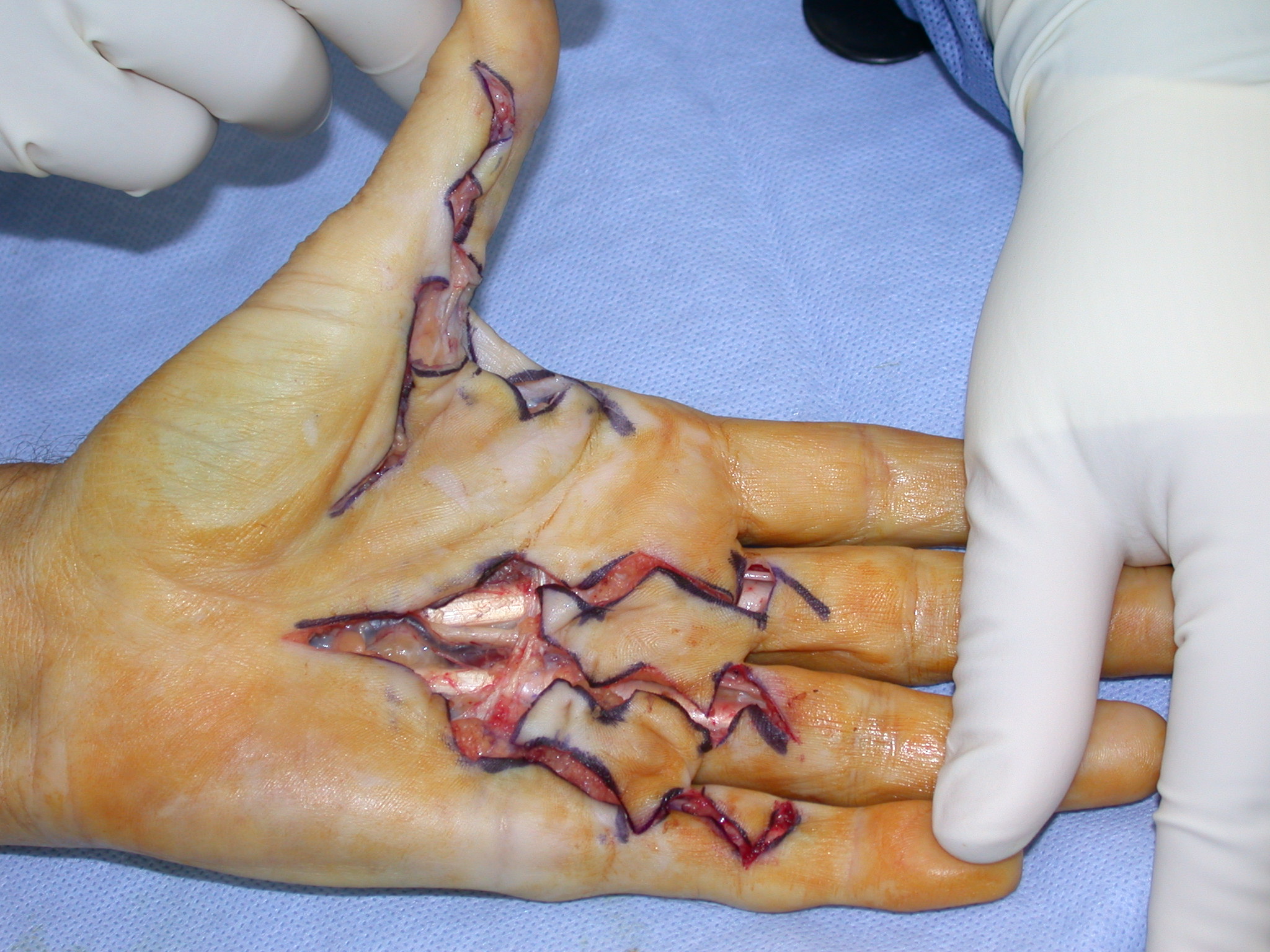
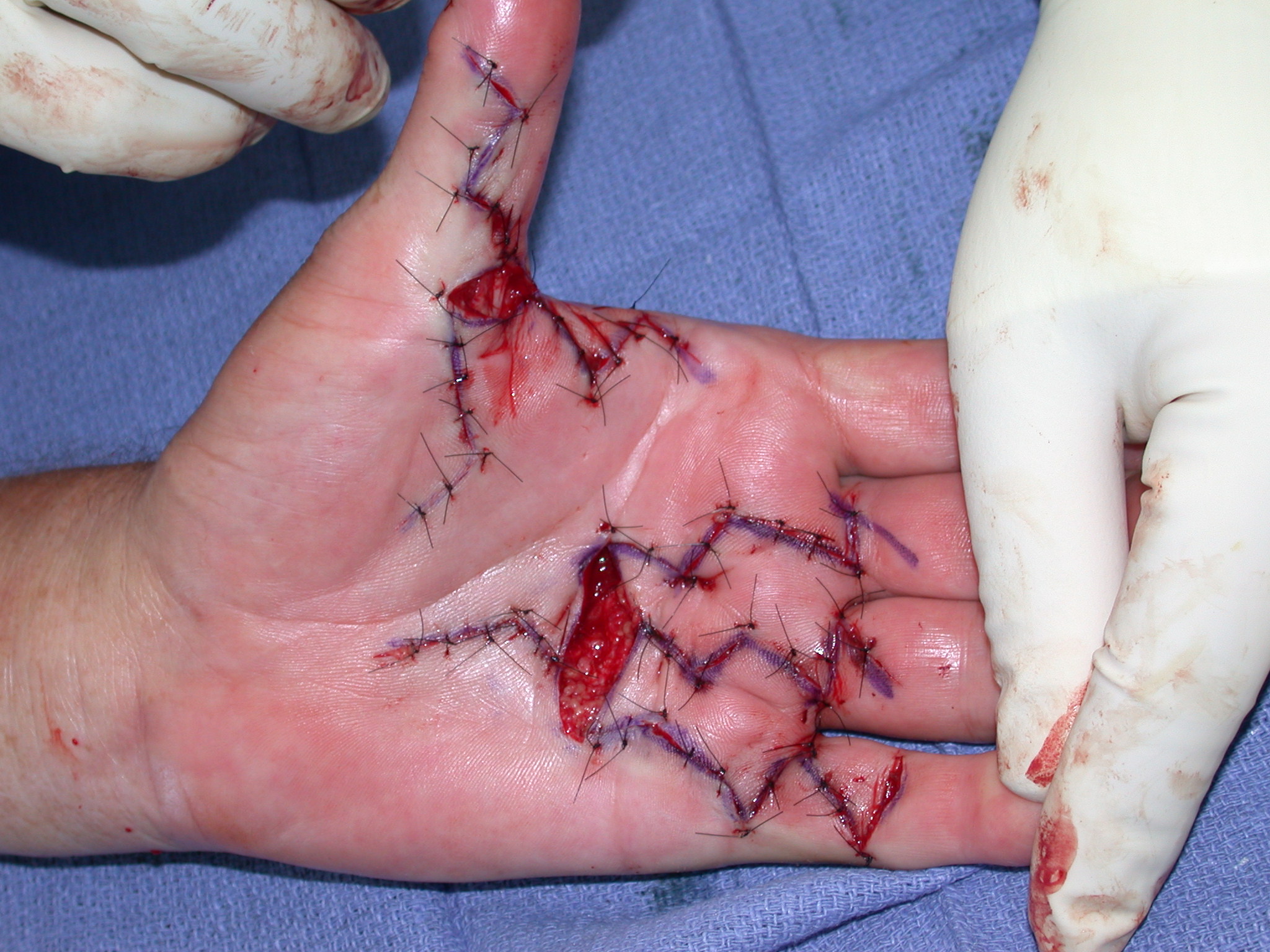
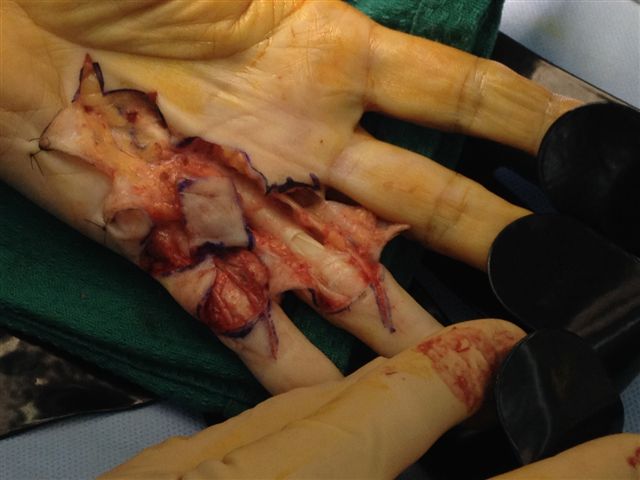
After the disease is excised and a joint release performed if necessary, as in the Surgical Technique section above, the wound is assessed for the best way of closure. The options for closure are very flexible with this technique, and need not be decided until the disease is excised and the wound assessed. The wound can sometimes be closed primarily (Figures 11a–d) or otherwise with Y-V advancement flaps (Figure 12) in which case the final scar more closely conforms with Littler’s diamond concept [13]. With the fingers in full extension the skin at the ends of each of the transverse incisions is assessed and any tension found is released by further extension of the incision. The skin flaps, particularly over the proximal phalanx, can be somewhat redundant, made so by the tissue expander effect of the bulk of the disease which has now been removed. These flaps can therefore be advanced in a Y-V fashion, thereby lengthening and de-tensioning the wound. Indeed even the flaps in the palm can be similarly advanced, albeit to a lesser extent. Any temptation to close the wound primarily under any degree of longitudinal tension must be vigorously resisted. The flaps are rather sutured (using 5.0 nylon or similar) where they lie comfortably, starting with apex sutures in the flaps. Some minor trimming of skin redundancy along the oblique edges of the flaps may be required. The transverse wounds are only sutured (and then using loose horizontal mattress sutures) if they are lying apposed with the finger in full extension. Otherwise they are left open to heal by secondary intention if the defect is less than 15 millimetres longitudinally, even if there are neurovascular bundles, tendon sheath or a small area of exposed tendon on view (Figures 13a–d). The open wounds heal relatively quickly with simple dressings and leave a fine scar (Figures 14a–b), as described by McCash in the palm [14] and by others in the digits, including Baron Guillaume Dupuytren himself [7], [15], [16]. Wound infections and haematomas are uncommon because the open wound allowed free drainage of any potential collection.
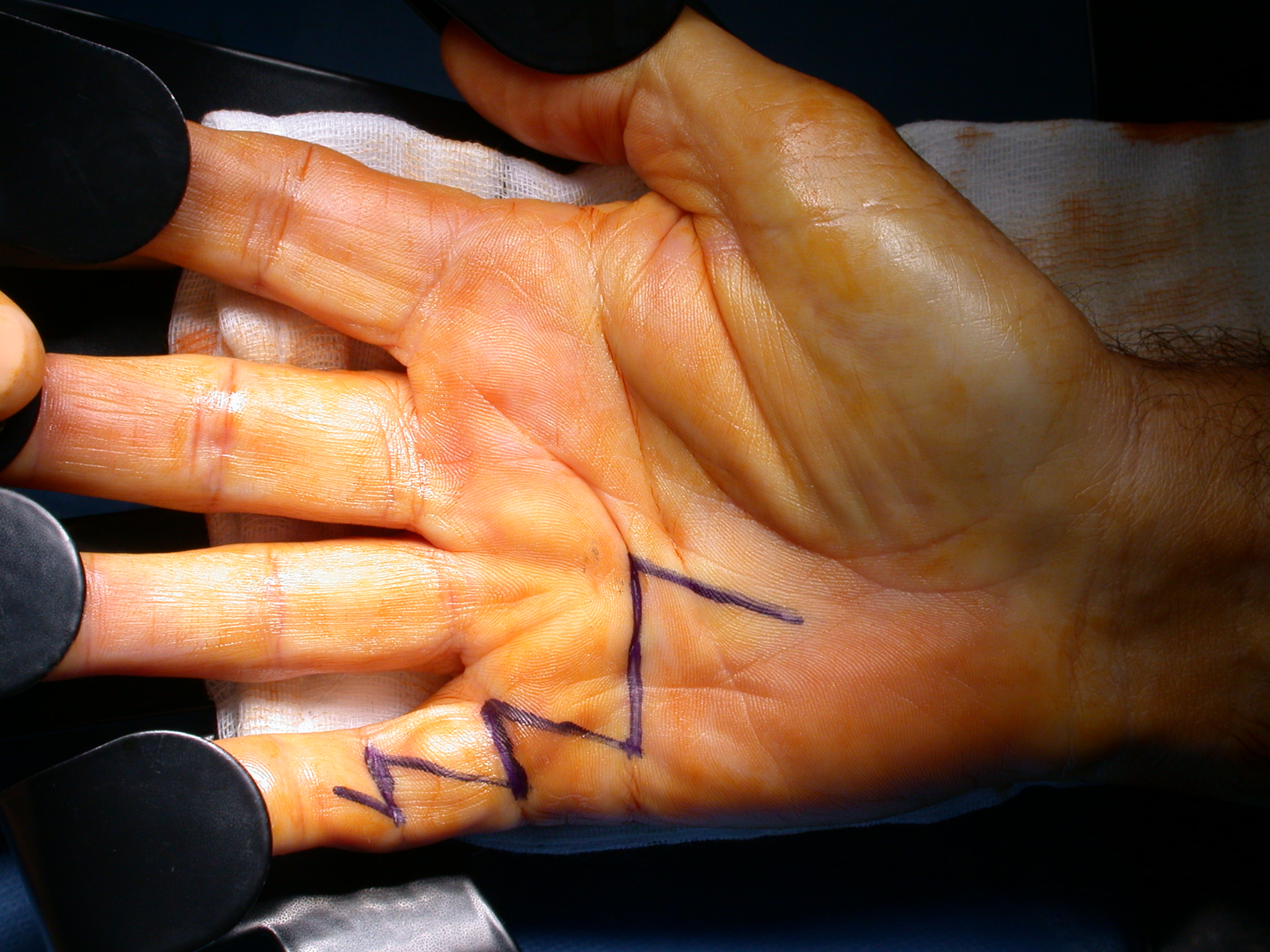
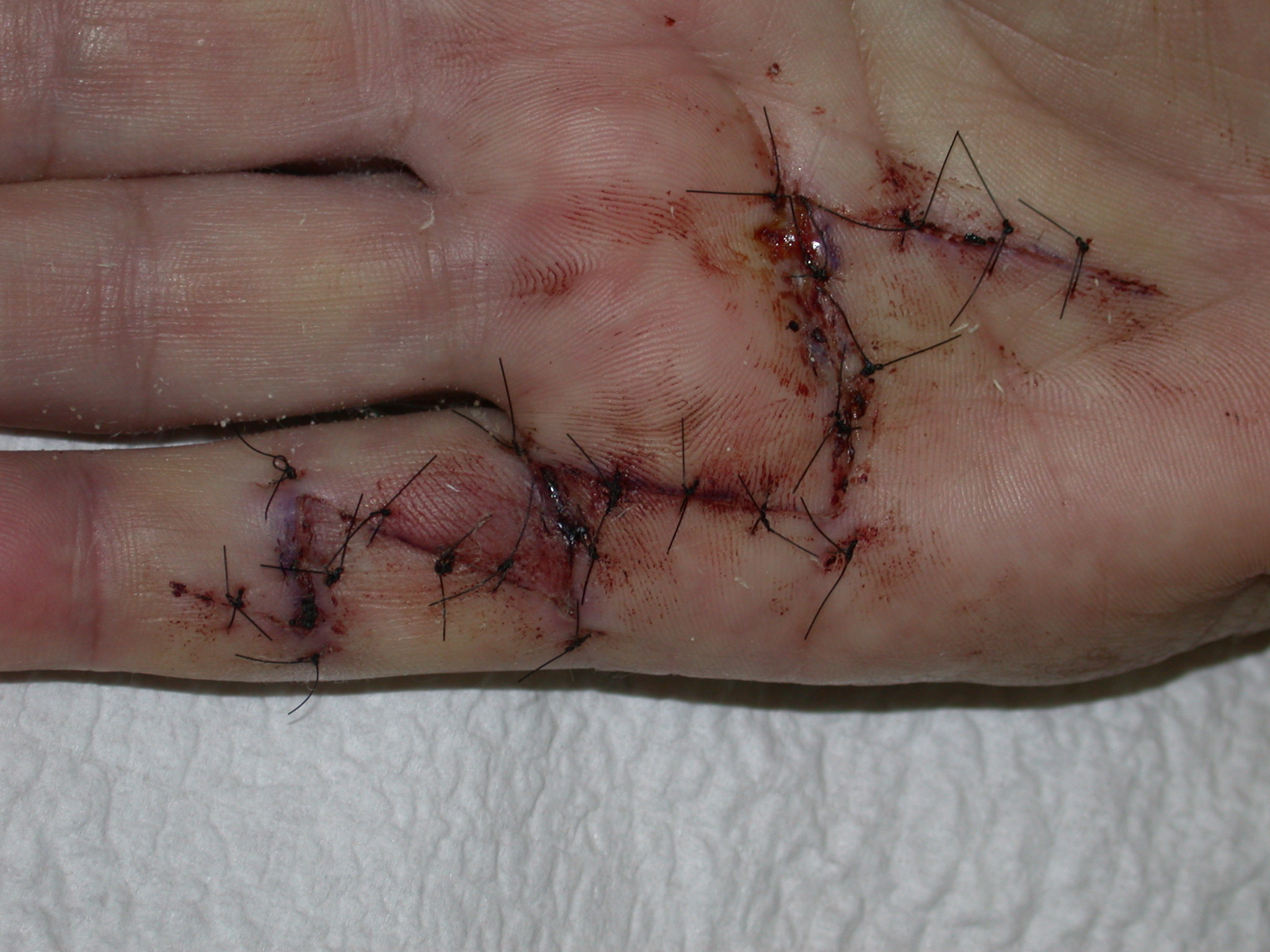
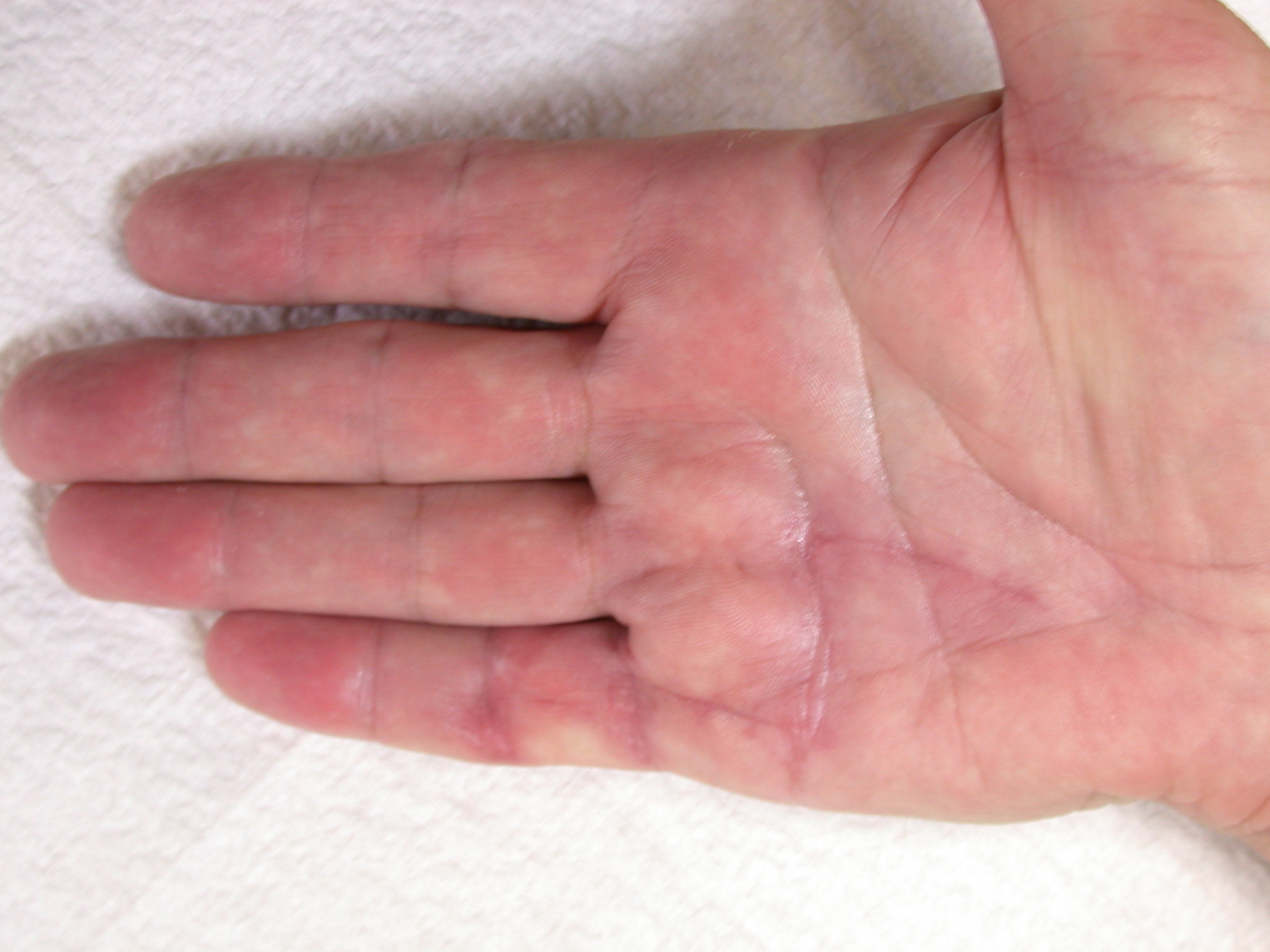
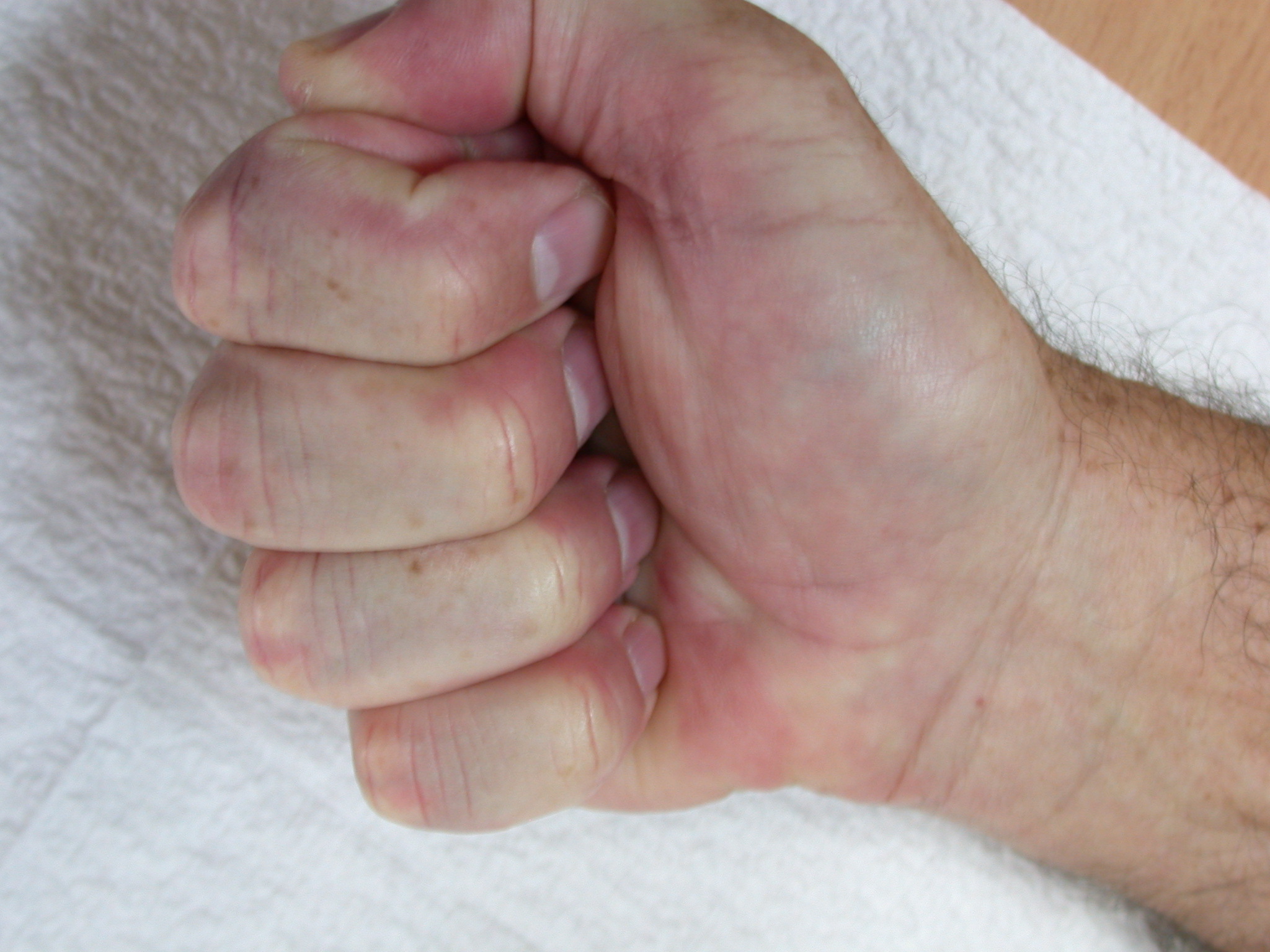
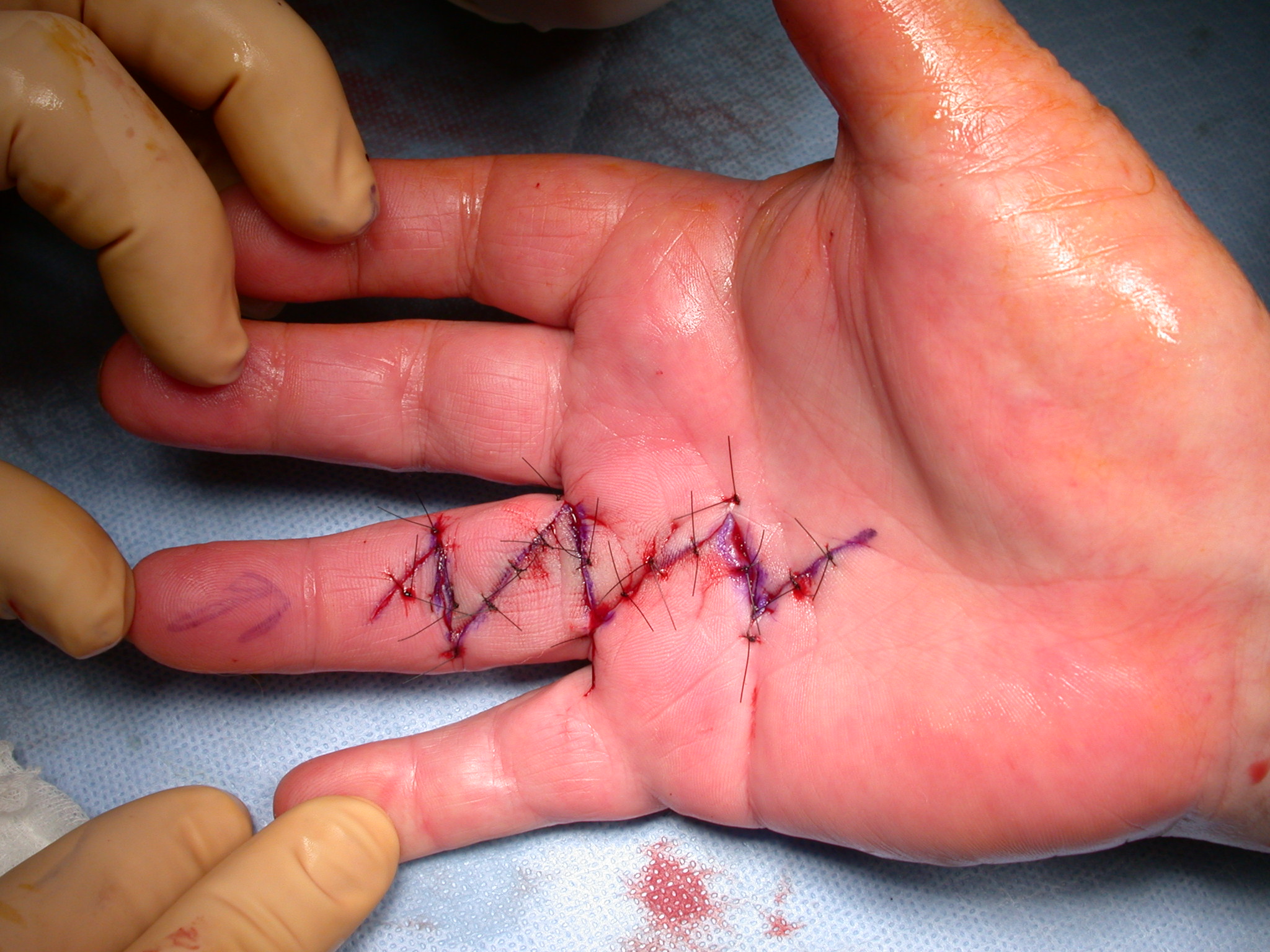
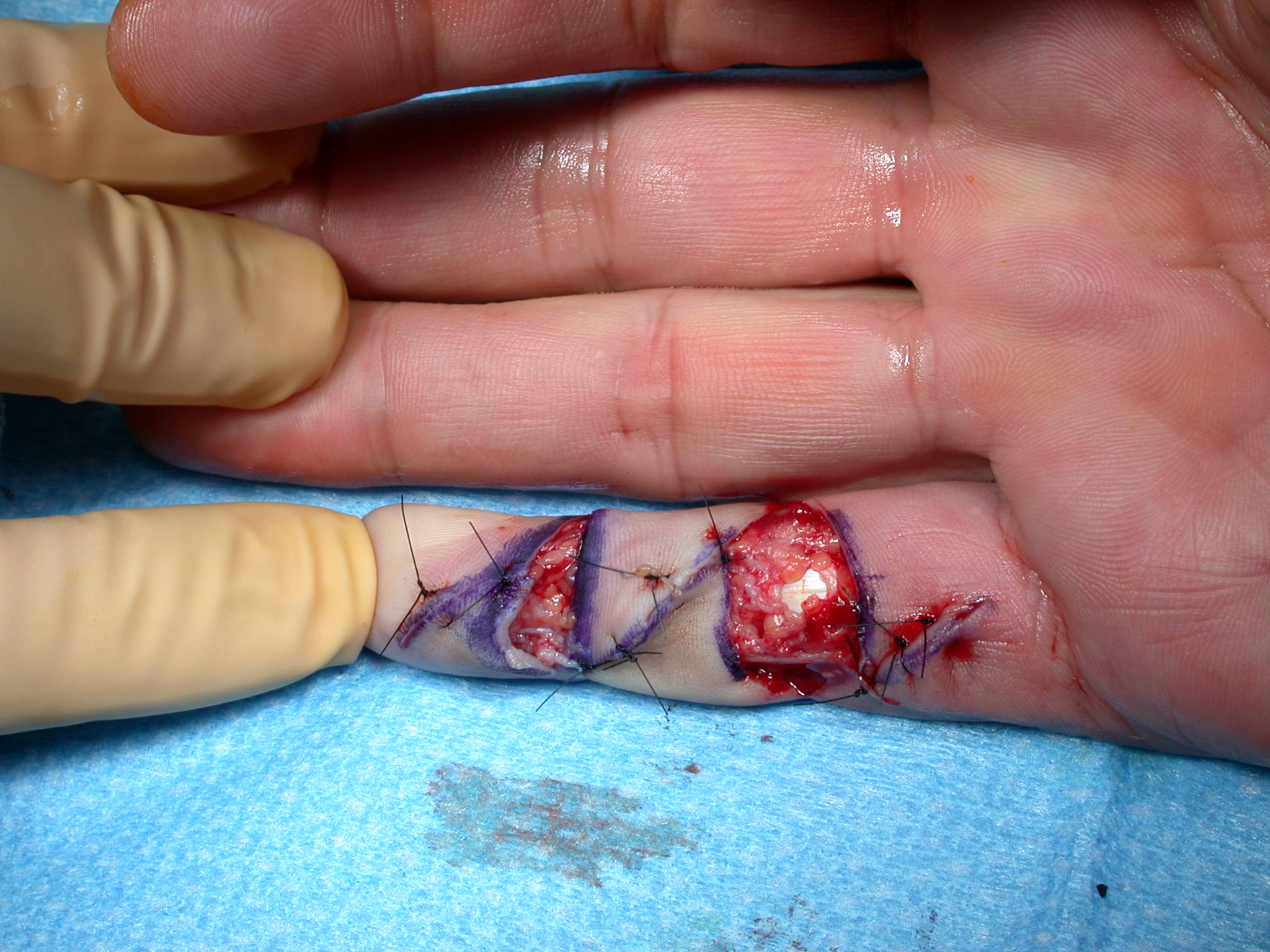
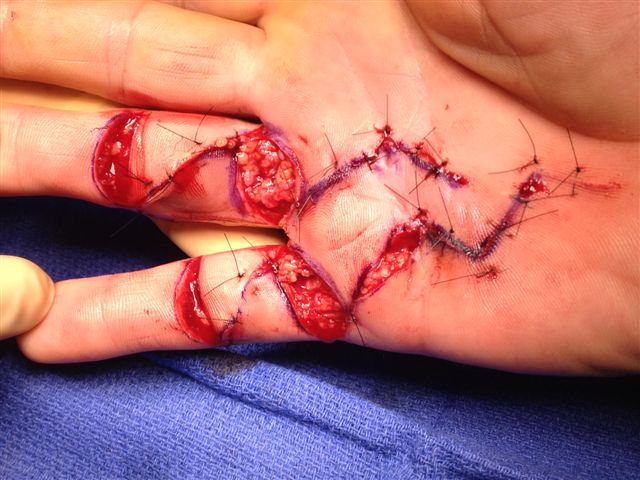
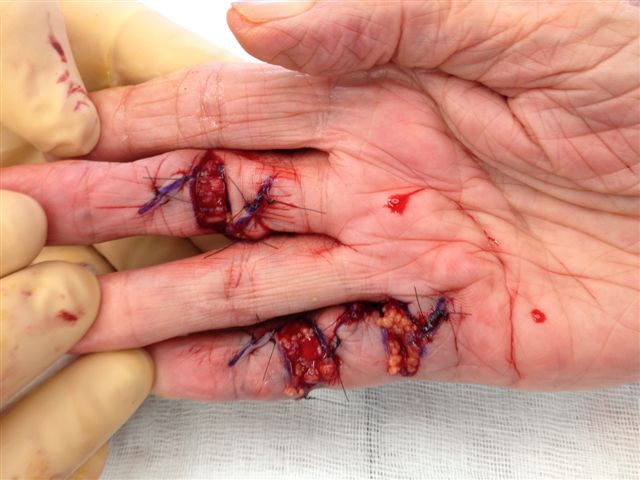
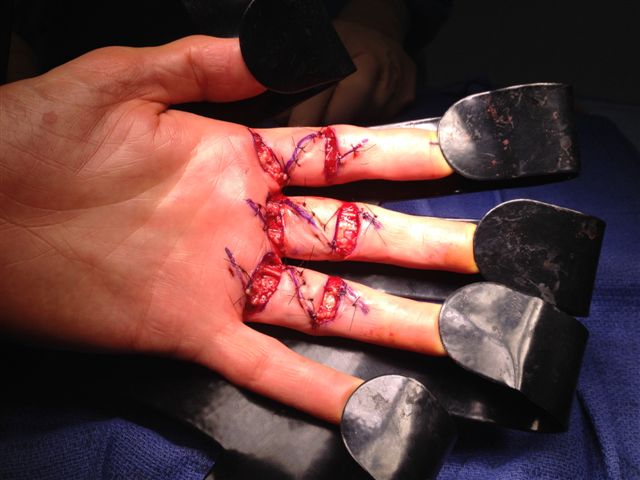
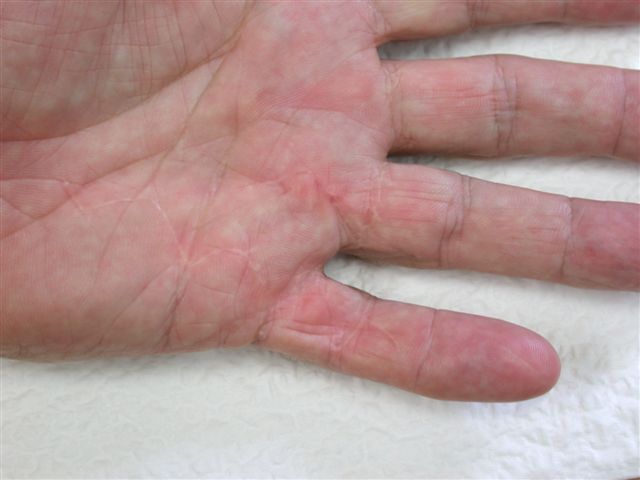
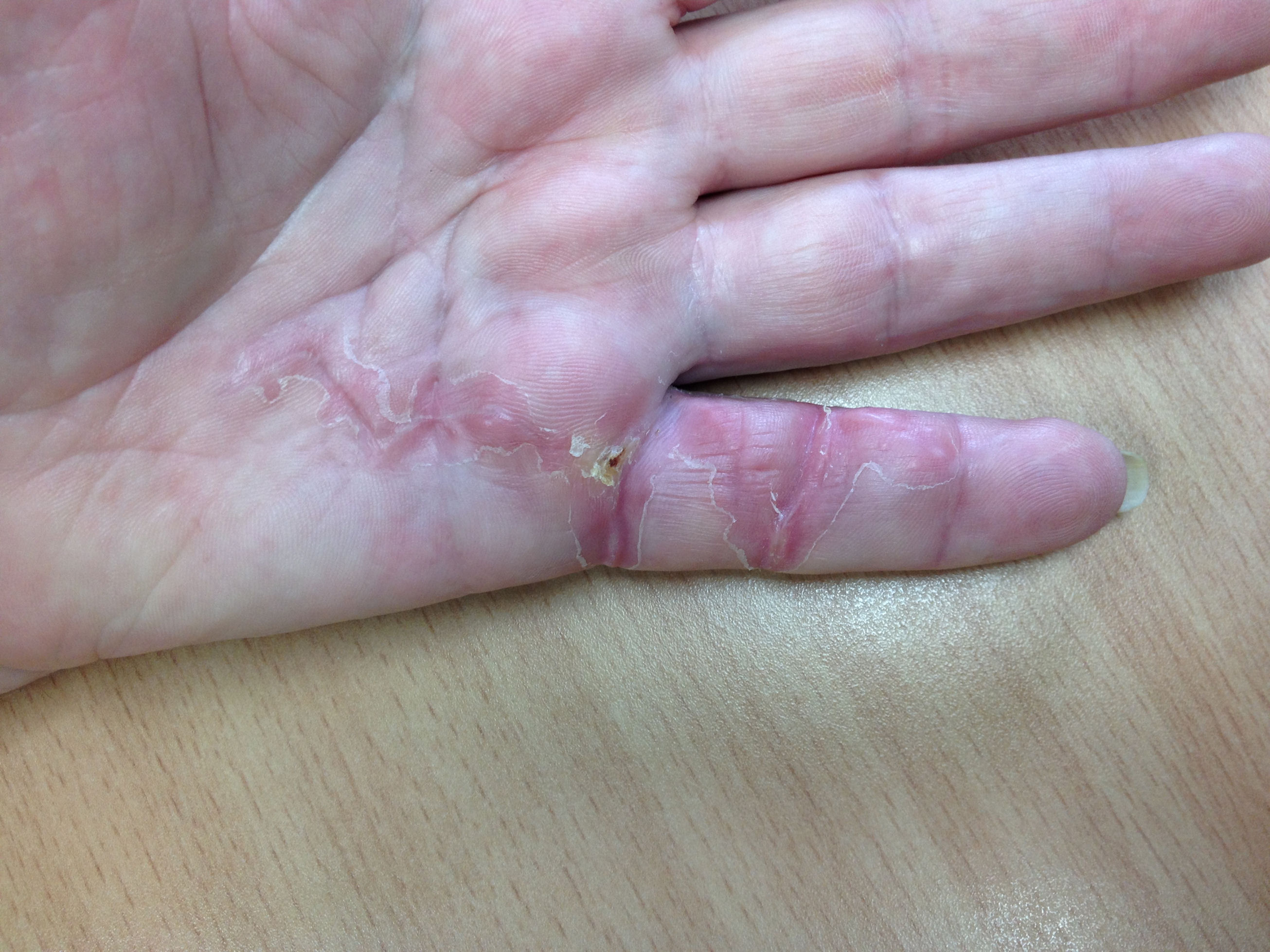
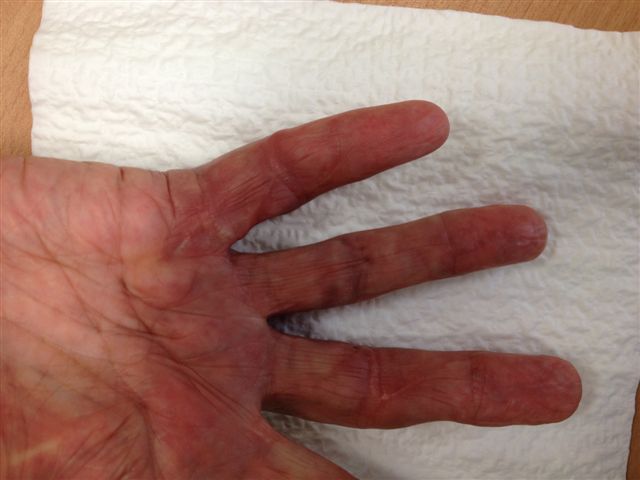
If the defect is greater than 15 millimetres longitudinally it is skin grafted with a FTSG (Figure 15) which is readily harvested using a transversely orientated excision centered on the cubital fossa crease.
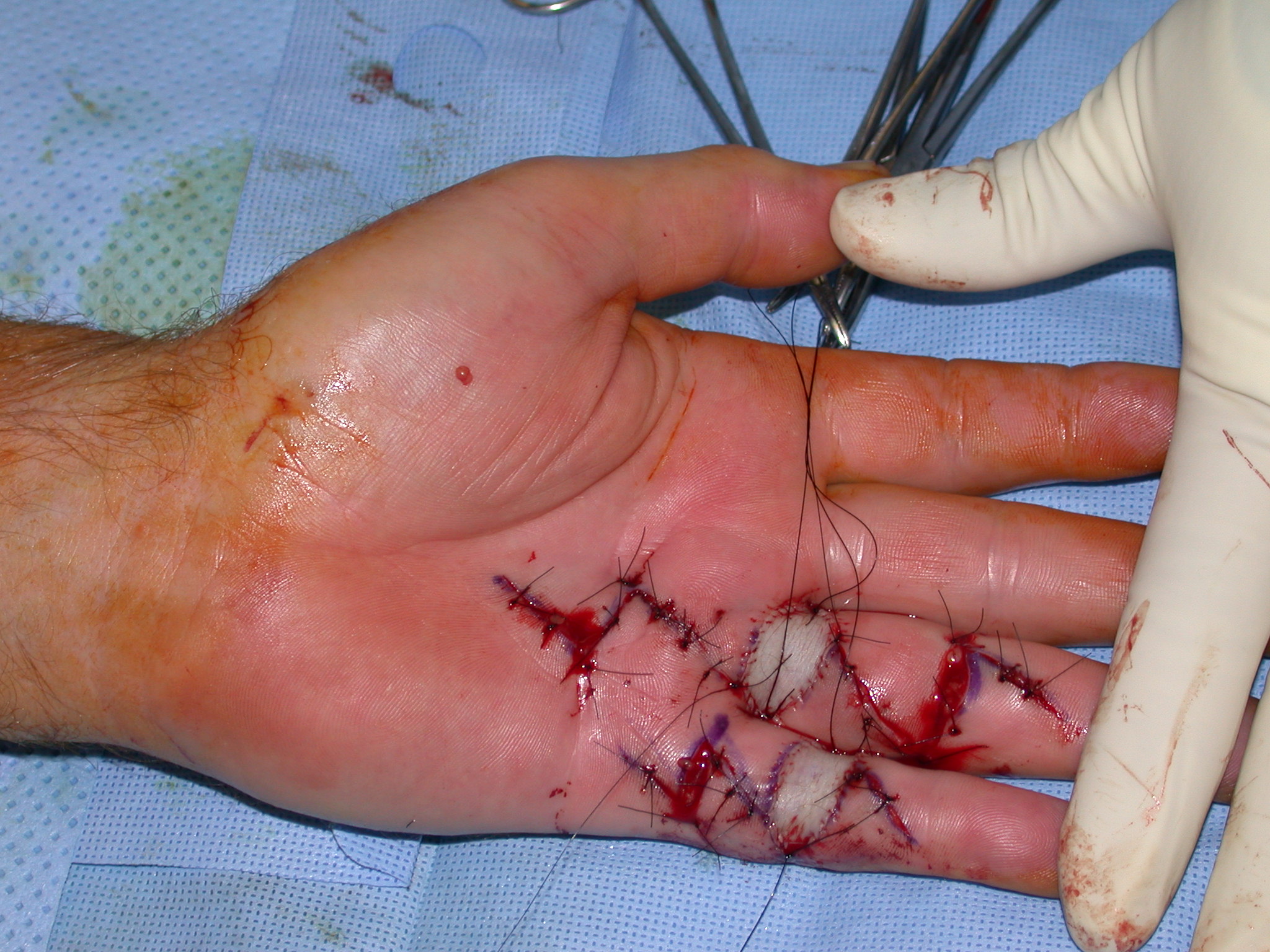
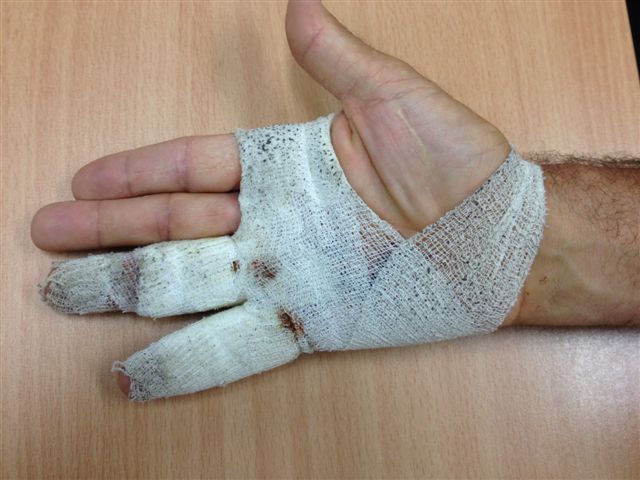
Dressings and a hand based plaster of Paris ulnar gutter splint are applied to hold the operated fingers in full extension. No drains are required if areas of the wound have been left open.
Post-operative care
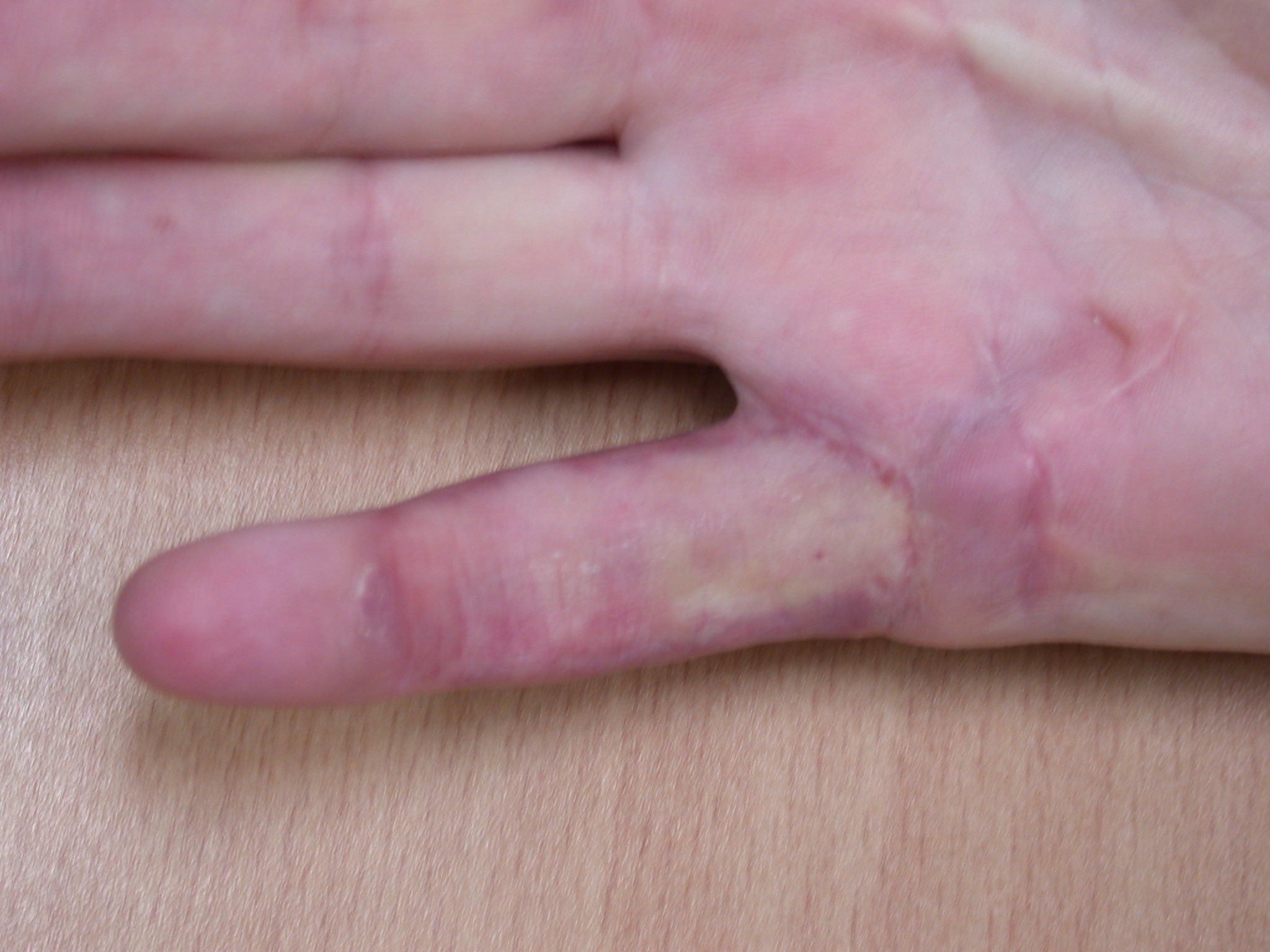
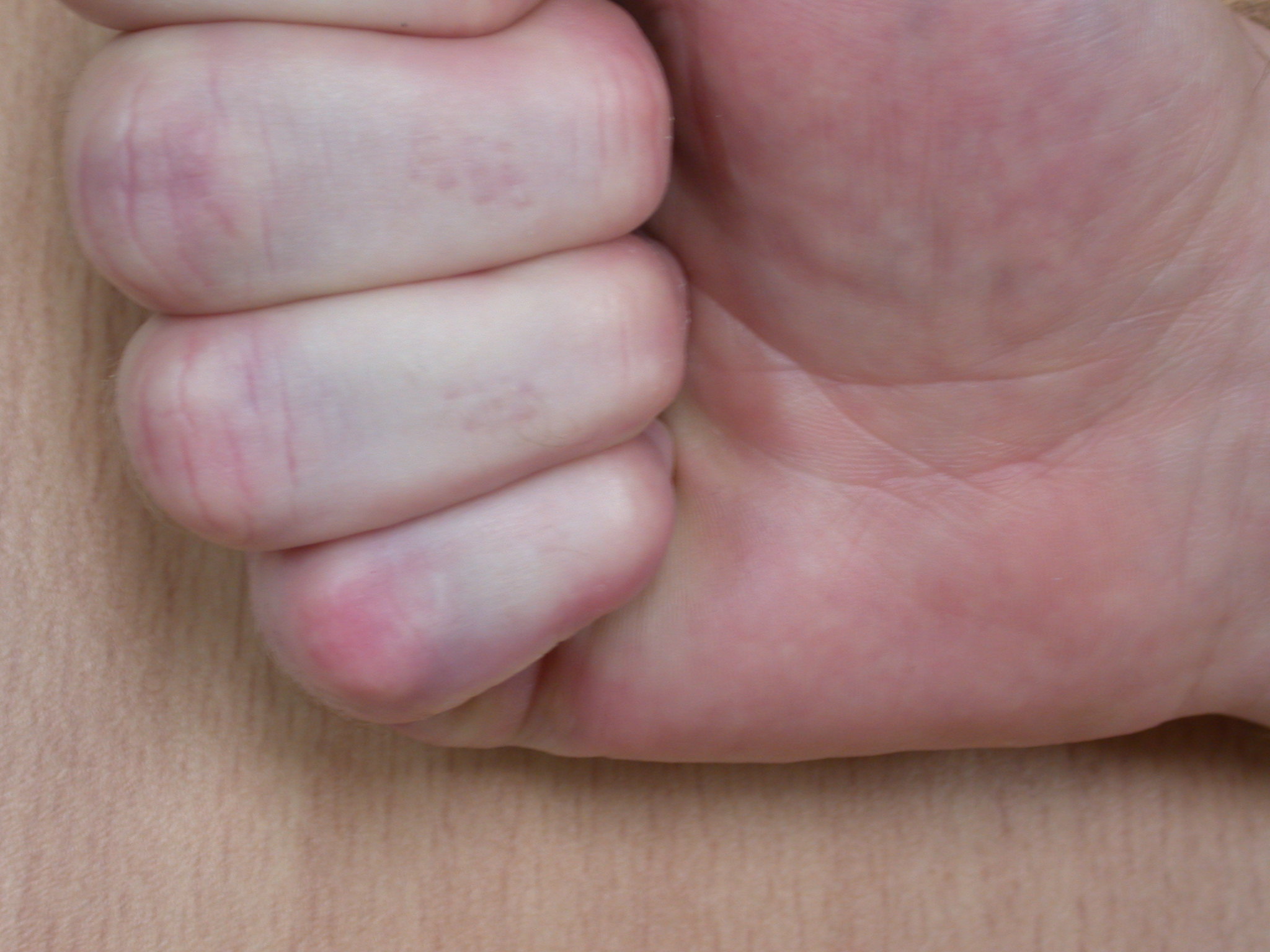
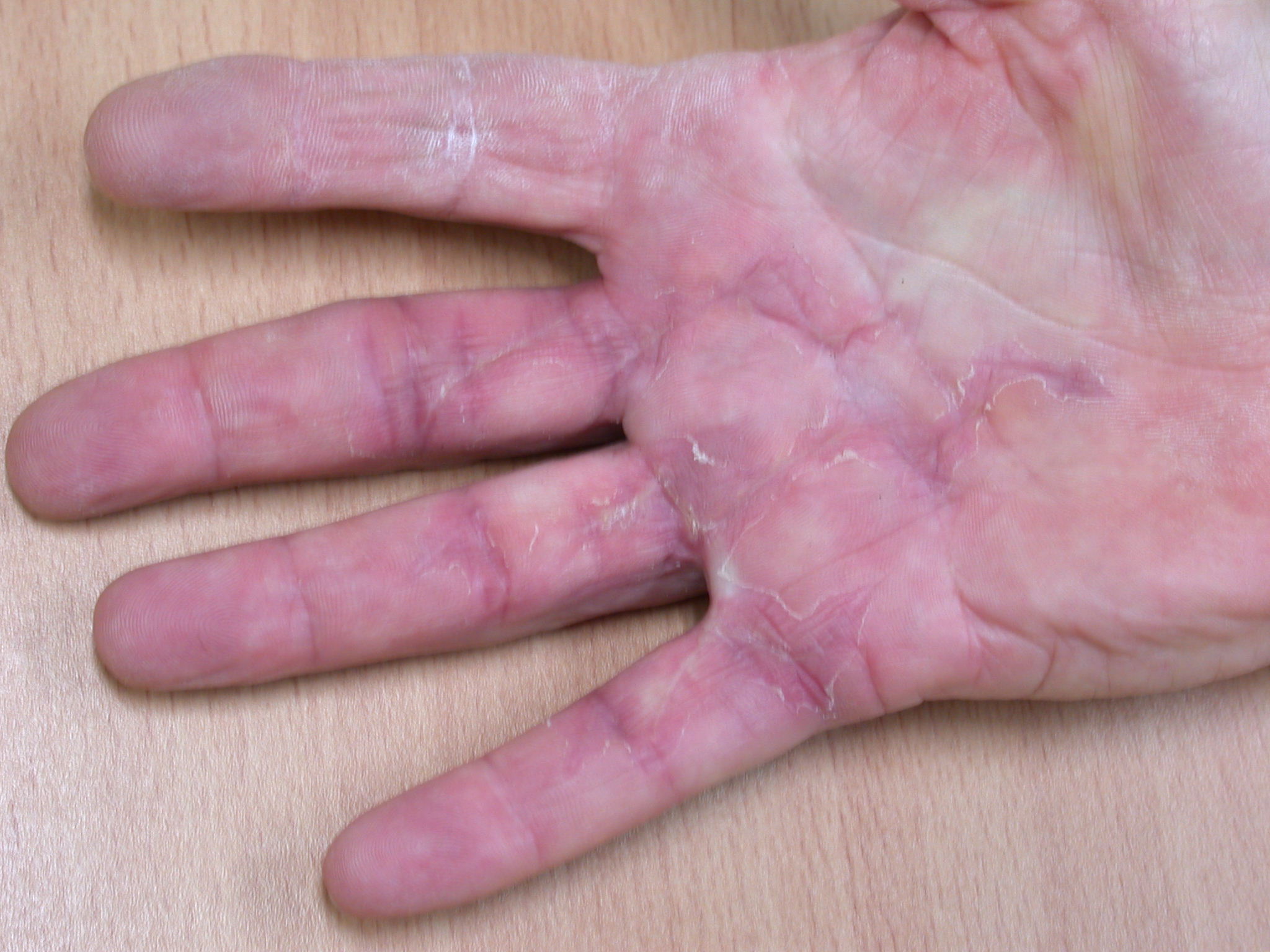
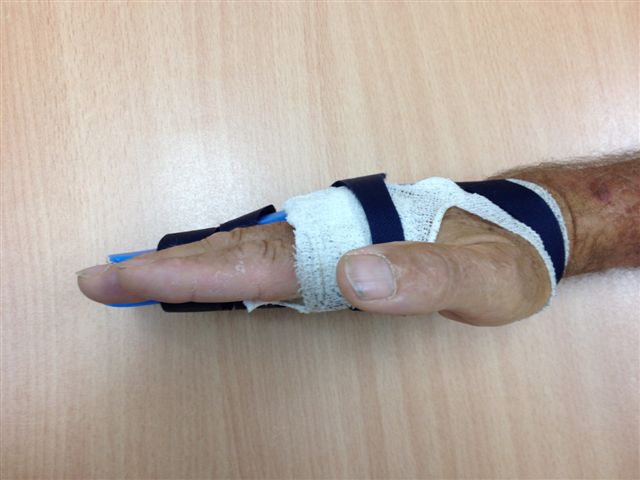
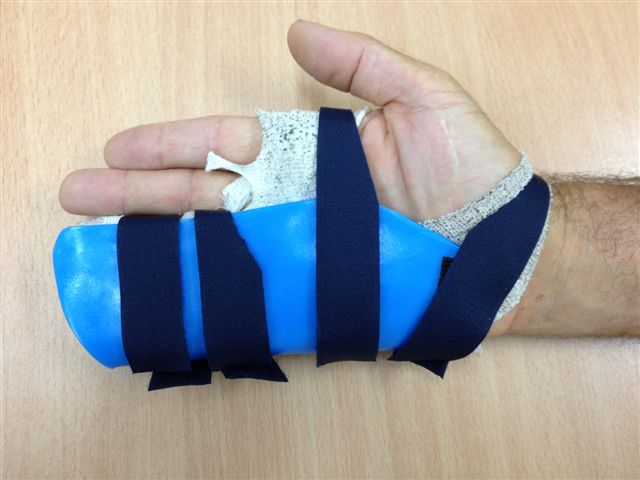
The surgical dressing is removed after five to seven days and a light dressing applied (Figure 16). Action and passive flexion and extension exercises are commenced, with the emphasis placed on maintaining full extension, especially of the PIPJ. A night extension splint is usually worn for six weeks to aid the maintenance of extension (Figures 17a–b) [2], although one systematic review suggests that this may be of doubtful effectiveness, at least in patients with severe contractures, and may delay the recovery of finger flexion [17]. A more recent study also found no benefit from postoperative splinting [18]. At twelve to fourteen days the sutures are removed and any open areas of the wound are covered with small non-adhesive dressings. Once wounds are healed sorbolene and vitamin E cream or similar can be massaged into the scars regularly and a silicone sheet can be applied at night to minimize scarring. An early recurrence of a PIPJ contracture can be reversed by use of finger based serial casts or a dynamic extension splint. Exercises are continued until full range of motion and strength are regained, after which a programme of ongoing occasional stretches is encouraged.
Results
One cannot hope nor expect to cure what is essentially a genetic disease with localized treatment. Additionally, all treatment modalities for Dupuytren’s Disease have a failure rate and a complication rate, including recurrence, and surgery is no exception.
In the literature the success of Dupuytren’s surgery is usually measured in terms of the degree of correction of the contracture and/or the improvement in range of motion, the recurrence rate and the complication rate. Sometimes, patient satisfaction and the results of formal hand function tests are also reported. In general:
- MCPJ contractures fare better than PIPJ contractures
- the risk of recurrence is less in older patients
- the risk of recurrence increases over time
- the results are worse with severe contractures
- little finger disease tends to be more difficult to correct and more inclined to recur
- revision surgery has poorer results than primary surgery
There is difficulty in comparing the results and complication rate from different studies due in large part to differences in the patient cohorts particularly in relation to the severity of the presenting disease. Also there are differences in the definitions of some outcomes, for example recurrence and infection, and the way those outcomes are determined, for example prospectively, by retrospective chart review or by patient questionnaire.
Studies looking at the results of fasciotomy give a mean improvement in contracture angle ranging from 46 to 88%, and a recurrence rate averaging 62% at about 4 years, although some investigators have included percutaneous needle fasciotomy in the fasciotomy group analysis [19].
Segmental fasciotomy has had good results reported by some, and this is possibly because resection of a short segment of disease in some cases may be sufficient to prevent short-term cord re-formation and de-tension the disease leading to involution. Shin et al achieved a 93% improvement at the MCPJ and an 82% improvement at the PIPJ, with no recurrence at 2 years [20]. Moermans reported a 38% recurrence rate at greater than 1 year in the 59% of the 240 patients available for follow-up [4]. The technique is however still challenging, and has the ever present risk of nerve injury, stiffness and wound complicatons [21] and other investigators have reported high recurrence rates [22].
Partial fasciectomy is probably the most commonly employed surgery for Dupuytren’s. With the surgery being more extensive, one might expect to see a higher rate of complete correction, and this is borne out in the literature with a 100% correction being reported in 61 to 97% of patients, and a mean improvement in contracture angle ranging from 58 to 79%, and a recurrence rate averaging 39% at 4 years [19]. One large study showed an improvement of 94 % in MCPJ contractures and of 72% in PIPJ contractures [23]. Surgery involving primary closure with local flaps yielded a 92% improvement in MCPJ contractures and a 56% improvement in PIPJ contractures [24]. One study found no difference between using a straight incison and closure with Z-plasties versus a Palmen incision [7] with multiple V-Y plasties [25].
Total fasciectomy is not a commonly used technique, although it has been advocated in a recent publication [26] which reported a correction rate for primary surgery ranging between 50 and 100% (depending on the initial severity) and a 13.8% complication rate.
Regardless of the operative approach, some authors have suggested that the use of full thickness skin grafts (even without the excision of any digital skin) can reduce the risk of recurrence [27]. It may well be that using skin grafts is effective primarily not because of the presence of the graft but because in doing so all longitudinal tension is taken out of the wound. Possibly further studies will show that leaving the wounds partially open achieves the same effect and similar results.
Dermofasciectomy can result in some problems with the skin grafts and with stiffness, but can give recurrence rates as low as 8% even in revision surgery [28]. Tonkin et al found a marked reduction in the recurrence of disease in patients who had had a skin graft compared to those who had not, with no additional loss of flexion [1], supporting the findings and teaching of Hueston from the 1960’s [29]. However this long held belief has been questioned by at least one more recent study which found no difference in correction or recurrence between patients having a Z-plasy closure and those having a dermofasciectomy and skin graft [30].
In situations where a capsuloligamentous joint release is required, there may be a trade off between achieving and maintaining full correction and minimizing stiffness. Releasing the PIPJ and splinting it for a period in extension will result in some delay in regaining the flexion range and strength, but incomplete correction of the contracture increases the recurrence rate [31]. Good results have been reported from releasing the PIPJ in the little finger when necessary [32] and in other digits besides [33].
Complications
The variety and incidence of complications following Dupuytren’s surgery is relatively high when compared with simpler hand operations. Virtually the whole gamut of surgical complications can be encountered including infection, haematoma, swelling, nerve injury, skin flap or digital ischaemia, delayed wound healing problems, hypertrophic scars, stiffness, weakness, granulomas, incomplete correction of contractures, recurrence of disease and complex regional pain syndrome [31], [34]. The most common early complications in one study were transient numbness, wound infection and circulatory disturbance [31]. Digital nerve injury has been reported around 4 to 5% and complex regional pain syndrome around 5 to 10% [35], [36]. Incomplete correction of contractures is also common in some studies, quoted as 31% by Dias et al. [31]. The incidence of most complications, as expected, generally increases with the severity of the disease [31], [37]. However nerve injury still occurs in less invasive surgery [20] and recurrence is less common with more extensive resection [31]. The over-all complication rate as report by patients in one study was 46% [31].
Recurrence rates vary between studies depending on not only factors relating to the treatment and the patients but also the length of follow-up and the definition of recurrence. Reported recurrence rates in one review paper range from 2 to 47% with the risk of recurrence being higher in younger patients, more severe contractures and incomplete correction of the deformity [31]. In another paper the recurrence rates range from 8 to 54% [28], and a systematic review found reported recurrence rates ranging from 0 to 71% [38].
Revision surgery
The indications for revision surgery are more conservative, given the more difficult nature of the surgery, the higher complication rate and the poorer results. Care must be taken in assessing the finger with a flexion contracture following Dupuytren’s surgery. Was the contracture fully corrected at the initial operation? Is the contracture progressing? Is there palpable recurrent Dupuytren’s disease? Is the PIPJ arthritic? Is the extensor tendon weak? What is the skin quality? What is the patency of the digital arteries as assessed by Allen’s test? Are the digital nerves intact? What is the range of flexion? All these factors will influence decision making regarding revision surgery. Revision surgery is much more difficult than primary surgery in large part because of loss of the normal tissue plane around the neurovascular bundles, which instead are often densely adherent to the surrounding tissue and very difficult to dissect out intact. In addition the skin quality is usually poor and the PIPJ is usually stiffer after resection of the diseased tissue. A joint release is more likely to be required and full thickness skin grafting is recommended.
Alternatives for the multi-operated digit include PIPJ fusion and finger amputation.
Conclusion
Surgery for Dupuytren’s contracture can give good results, but it is challenging and has a relatively high complication rate. There is a range of surgical options available, with no single approach being clearly better than any other.
References
[1] Tonkin MA, Burke FD, Varian JP. Dupuytren’s contracture: a comparative study of fasciectomy and dermofasciectomy in one hundred patients. J Hand Surg Br. 1984;9:156-62. DOI: 10.1016/S0266-7681(84)80018-2[2] Au-Yung ITH, Wilden CJ, Page RE. A review of common practice in Dupuytren Surgery. Tech Hand Upper Extrem Surg. 2005;9(4):178-87.
[3] Milner RH. Dupuytren’s disease affecting the thumb and first web of the hand. J Hand Surg Br. 2003;28(1):33-6. DOI: 10.1054/jhsb.2002.0840
[4] Moermans JP. Long-term results after segmental aponeurectomy for Dupuytren’s disease. J Hand Surg Eur Vol. 1996;21B:797-800.
[5] Bruner JM. The zigzag volar-digital incision for flexor tendon surgery. Plast Reconstr Surg. 1967;40:571.
[6] King EW, Bass DM, Watson HK. Treatment of Dupuytren’s contracture by extensive fasciectomy through multiple Y-V-plasty incisions: short-term evaluation of 170 consecutive operations. J Hand Surg Am. 1979;4:234–41. DOI: 10.1016/S0363-5023(79)80158-6
[7] Tubiana R, Leclercq C, Hurst LC, Badalamente MA, Mackin EJ. Dupuytren’s Disease. London: Martin Dunitz; 2000. p. 7, 134, 140.
[8] Hettiaratchy S, Tonkin MA, Edmunds IA. Spiralling of the neurovascular bundle in Dupuytren’s disease. J Hand Surg Eur Vol. 2010;35(2):103-8. DOI: 10.1177/1753193409349855
[9] Meathrel KE, Thoma A. Abductor digiti minimi involvement in Dupuytren’s contracture of the small finger. J Hand Surg Am. 2004;29(3):510-3.
[10] Crowley B, Tonkin MA. The proximal interphalangeal joint in Dupuytren’s disease. Hand Clin. 1999;15(1):137-47.
[11] Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH. Green’s Operative Hand Surgery. 6th ed. Edinborough: Churchill Livingstone; 2011.
[12] Edmunds I, Chien C. A new surgical approach to Dupuytren’s disease. J Hand Surg Eur Vol. 2011;36(6):485-9. DOI: 10.1177/1753193411404338
[13] Littler JW. Hand, wrist and forearm incisions. In: Littler JW, Cramer LM, Smith JW, editors. Symposium on reconstructive hand surgery. St Louis: CV Mosby; 1974. p. 87–97.
[14] McCash CR. The open palm technique in Dupuytren’s contracture. Br J Plast Surg. 1964;17:271-80. DOI: 10.1016/S0007-1226(64)80043-6
[15] Foucher G, Cornil C, Lenoble E. Open palm technique for Dupuytren’s disease. Ann Chir Main (Ann Hand Surg). 1992;11:362–6.
[16] Elliot D. Pre-1900 literature on Dupuytren’s disease. Hand Clin. 1999;15:175–81.
[17] Larson D, Jerosch-Herold C. Clinical effectiveness of post-operative splinting after surgical release of Dupuytren’s contracture: a systematic review. BMC Musculoskelet Disord. 2008;9:104. DOI: 10.1186/1471-2474-9-104
[18] Kemler MA, Houpt P, van der Horst CMAM. A pilot study assessing the effectiveness of postoperative splinting after limited fasciectomy for Dupuytren’s disease. J Hand Surg Eur Vol. 2012;37(8):733-7. DOI: 10.1177/1753193412437631
[19] Crean SM, Gerber RA, Hellio Le Graverand MP, Boyd DM, Capperelleri JC. The effectiveness and safety of fasciectomy and fasciotomy for Dupuytren’s contracture in European patients: a structured review of the literature. J Hand Surg Eur Vol. 2014;36E(5):396-407. DOI: 10.1177/1753193410397971
[20] Shin EK, Jones NF. Minimally invasive technique for release of Dupuytren's contracture: segmental fasciectomy through multiple transverse incisions. Hand (NY). 2011 Sep;6(3):256-9. DOI: 10.1007/s11552-011-9336-6
[21] Andrews JG, Kay NRM. Segmental aponeurectomy for Dupuytren’s disease: a prospective study. J Hand Surg Eur Vol. 1991;6(3):255-7.
[22] Gelman S, Schlenker R, Bachoura A, Jacoby SM, Lipman J, Shin EK, Culp RW. Minimally invasive partial fasciectomy for Dupuytren's contractures. Hand (N Y). 2012 Dec;7(4):364-9. DOI: 10.1007/s11552-012-9461-x
[23] Coert JH, Nerin JPB, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57(1):13-7. DOI: 10.1097/01.sap.0000205819.53215.52
[24] Uemura T, Kazuki K, Egi T, Yoneda M, Takamatsu K, Nakamura H. Clinical outcomes of primary skin closure with Y-V and Z-plasties for Dupuytren’s contracture: use of one stage skin closure. J Plast Surg Hand Surg. 2010;44(6):306-10. DOI: 10.3109/2000656X.2010.534340
[25] Citron ND, Nunez V. Recurrence after surgery for Dupuytren’s disease: a randomized trial of two skin incisions. J Hand Surg Br. 2005;30(6):563-6.
[26] Hogeman A, Wolfhard U, Kendoff D, Olivier LC. Results of total aponeurectomy for Dupuytren’s disease in 61 patients: a retrospective clinical study. Arch Orthop Trauma Surg. 2009;129(2):195-201. DOI: 10.1007/s00402-008-0657-z
[27] Roy N, Sharma D, Mirza AH, Fahmy N. Fasciectomy and conservative full thickness grafting in Dupuytren’s contracture: The fish technique. Acta Orthop Belg. 2006;72(6):678-82.
[28] Hall PN, Fitzgerald A, Sterne GD, Logan AM. Skin replacement in Dupuytren’s disease. J Hand Surg Eur Vol. 1997;22(2):193-7.
[29] Hueston J. The control of recurrent Dupuytren’s contracture by skin replacement. Br J Plast Surg. 1969;22:152-6. DOI: 10.1016/S0007-1226(69)80057-3
[30] Ullah AS, Dias JJ, Bhowal B. Does a ‘firebreak’full-thickness skin graft prevent recurrence after surgery for Dupuytren’s contracture?: a prospective randomized trial. J Bone Joint Surg Br. 2009;91(3):374-8. DOI: 10.1302/0301-620X.91B3.21054
[31] Dias JJ, Braybrooke J. Dupuytren’s contracture: An audit of the outcomes of surgery. J Hand Surg Eur Vol. 2006;31B(5):514-21. DOI: 10.1016/J.JHSB.2006.05.005
[32] Ritchie JFS, Venu KM, Pillai K, Yanni DH. Proximal interphalangeal joint release in Dupuytren’s disease of the little finger. J Hand Surg Br. 2004;29(1):15-7.
[33] Beyermann K, Prommersberger KJ, Jacobs C, Lanz UB. Severe contracture of the proximal interphalangeal joint in Dupuytren's disease: does capsuloligamentous release improve outcome? J Hand Surg Br. 2004 Jun;29(3):240-3.
[34] Prosser R, Conolly WB. Complications following surgical treatment for Dupuytren’s contracture. J Hand Therapy. 1996;9:344-8. DOI: 10.1016/S0894-1130(96)80040-8
[35] Mavrogenis AF, Spyridonos SG, Ignatiadis IA, Antonopoulos D, Papagelopoulos PJ. Partial fasciectomy for Dupuytren’s contractures. J Orthop Adv. 2009;18(2):106-10.
[36] Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty. 2011;10:e15.
[37] Bulstrode NW, Jemec B, Smith PJ. The complications of Dupuytren’s contracture surgery. J Hand Surg Am. 2005;30(5):1021-5.
[38] Becker GW, Davis TRC. The outcome of surgical treatments for primary Dupuytren’s disease – a systematic review. J Hand Surg Eur Vol. 2010;35(8):623-6. DOI: 10.1177/1753193410376286



