Therapeutic strategies for uncomplicated cystitis in women
Jakhongir F. Alidjanov 2
Reinhard Fünfstück 3
Walter L. Strohmaier 4,5,6
Jennifer Kranz 7,8
Tommaso Cai 9
Adrian Pilatz 10
Florian M. E. Wagenlehner 10
1 Technical University of Munich, Straubing, Germany
2 Biostatistics & Data Science R&D, Bionorica SE, Neumarkt, Germany
3 Internal medicine, Sophien-und Hufeland-Klinikum Weimar/Gesundheitszentrum Weimar, Jena, Germany
4 Medical School Regiomed, Coburg, Germany
5 Julius Maximilian University, Wuerzburg, Germany
6 University of Split, Croatia
7 Department of Urology and Pediatric Urology, University Medical Center RWTH Aachen, Germany
8 Department of Urology and Kidney Transplantation, Martin Luther University, Halle (Saale), Germany
9 Dept. of Urology, Santa Chiara Regional Hospital, Trento, Italy
10 Clinic for Urology, Pediatric Urology and Andrology, Justus Liebig University of Giessen, Giessen, Germany
Abstract
Uncomplicated cystitis is affecting many women of all ages and has a great impact on the quality of life, especially in women suffering from recurrent, uncomplicated cystitis. By far the most frequent uropathogen, E. coli, may have acquired increasing resistance against a variety of oral antibiotics, which may differ between countries and regions. Therefore, local resistance data are important to be considered. On the other hand, non-antibiotic therapy has also become an option which should be discussed and offered to the patient. In patients suffering from recurrent uncomplicated cystitis, individual risk factors and possible behavioral changes should first be taken into account. Non-antimicrobial prophylactic strategies shown to be successful in well-designed clinical studies are the next options. Long term antibiotic prophylaxis, however, should only be considered as a last option. For some of those patients self-diagnosis and self-treatment may be suitable, e.g. by using a recognized questionnaire.
1 Epidemiology
Uncomplicated acute cystitis (uAC), also referred to as uncomplicated urinary tract infection (uUTI), is generally defined as an infection of the bladder in non-pregnant women with no known functional or anatomical abnormalities or co-morbidities [1]. These are distinguishable from acute pyelonephritis (an upper urinary tract infection) and complicated urinary tract infections (cUTI). The latter are a heterogeneous group of conditions and include those occurring in male and female patients with certain co-morbidities and abnormalities that impact urological function, and also include healthcare-associated and systemic infections [2].
It is well established that uUTIs are common in female patients of all ages, with an annual prevalence of ~11%, and are more common than cUTIs [2], [3]. Up to 80% of females will experience at least one uUTI in their lifetime, and as many as 45% will have recurrent uUTIs [4], [5], [6], [7]. Given their prevalence, uUTIs represents a substantial burden – without prompt and effective treatment, symptoms can be debilitating for several days and can impact work and daily routines [8], [9], [10].
The primary need of patients with uUTI is an accurate and early diagnosis followed by timely symptom relief. Current guidelines recommend empirical prescribing of selected antimicrobial agents [1], [11], [12], [13], [14], [15], which remains a largely effective approach for the acute episode. In young women experiencing a first episode of uUTI symptoms, urine culture is not recommended when a robust diagnosis can be reached by patient history-taking and other potential causes of symptoms can be excluded, which is important in order to minimize overdiagnosis and inappropriate treatment.
Indeed, uUTIs are one of the most common conditions associated with antimicrobial prescription [16], [17], and previous antibiotic exposure is associated with an increased risk of antimicrobial resistance (AMR), which may therefore present a public-health challenge [18], [19]. In particular, AMR of common uropathogens, e.g. E. coli, to therapies widely used for the management of uUTI, such as fluoroquinolones, is increasing in many regions [20]. Fluoroquinolones also transiently suppress commensal intestinal Enterobacteriaceae, associated with the development of AMR, and resistant strains can then spread to unexposed household contacts of patients treated with fluoroquinolones for urinary tract infection (UTI) [21]. Consequently, there is a need for novel oral therapies with activity against resistant strains of uropathogens, including extended-spectrum β-lactamase(-ESBL)-producing E. coli that are becoming more prevalent worldwide [22], [23], [24], [25], [26].
The safety of antimicrobial therapy is also a major concern. In recent years, the U.S. Food and Drug Administration (FDA) has published warnings regarding the use of fluoroquinolones for infections such as uAC [27], [28]. In particular, potential severe adverse effects on multiple organ systems, mentation and glucose control have resulted in recommendations that these drugs should not be prescribed for uAC unless there are no other alternatives [1], [11]. Therefore, alternative, non-antibiotic therapy for uAC should be considered and appropriate non-antibiotics or phytotherapeutics should be tested for equivalence with antibiotic therapy in well-designed clinical studies. Recurrent uUTIs are a major issue for many women and are associated with multiple visits to various healthcare professionals [29] and often repeated antibiotic prescriptions, with an increased risk of potential side effects [30], [31]. Some women may therefore prefer to avoid repeated courses of antibiotics and seek other treatment options [32].
| Diagnosis |
|
| Treatment of acute uUTI episodes |
|
| Prevention of recurrent uUTIs |
|
| Guidelines |
|
| Antimicrobial resistance (AMR) |
|
| Clinical trial design |
|
| Novel methods of consultation |
|
An expert panel that included urologists, obstetricians/gynecologists, infectious diseases specialists, emergency medicine specialists, clinical microbiologists and primary care physicians representing a broad geographical spread (Europe, North America, Latin America and Asia) has summarized and discussed the different topics which still need to be investigated more carefully in order to help better the vast number of our patients suffering from acute episodes of uAC (Table 1) [33].
2 Diagnosis of uncomplicated acute cystitis
Despite numerous publications, there is still no generally accepted strategy regarding the clinical diagnosis of uAC. The updated guidelines of the Infectious Diseases Society of America (IDSA) and the European Society for Microbiology and Infectious Diseases (ESCMID) mainly consist of recommendations about the treatment of uAC and not the diagnosis [11]. These guidelines were limited to the treatment of uAC and pyelonephritis in premenopausal, non-pregnant women with no known urological abnormalities or comorbidities. In addition, the authors noted that postmenopausal women or those who have well-controlled diabetes mellitus in the absence of urological sequelae may be considered as having uUTI by some experts, but a discussion of specific management of these groups was outside the scope of these guidelines.
In the last update of these guidelines of the European Association of Urology (EAU) from 2024, uAC is defined as acute, sporadic or recurrent cystitis limited to non-pregnant women with no known relevant anatomical and functional abnormalities within the urinary tract or comorbidities [1]. According to the EAU guidelines, the diagnosis of uAC can be made with a high probability based on a focused history of lower urinary tract symptoms (e.g. dysuria, frequency and urgency) and the absence of vaginal discharge or irritation.
The definition of UTIs in a broader sense is presented in the updated German National Clinical Practice S3 Guideline [34]: UTIs may be classified as uncomplicated in the absence of relevant functional or anatomical abnormalities in the urinary tract, with no relevant renal functional impairment and any relevant concomitant disease that could aggravate the UTIs or condition, which could increase the risk of development of serious complications. UAC in this regard may represent no additional health problem for the woman with stable diabetes mellitus, whereas any kind of pyelonephritis, whether earlier defined as uncomplicated or complicated, could interfere with her metabolic balance and could lead to severe complications.
It becomes obvious today that a simple general classification of UTIs into uncomplicated and complicated UTIs is far too rough. Therefore, a more differentiated stratification of UTIs with deeper consideration of risk factors was proposed earlier [35].
The U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) have proposed their guidelines for the clinical diagnosis of patients with uAC for clinical studies as follows:
- Adult and, if appropriate, adolescent females with evidence of pyuria (WBC≥10/μL) and at least two of the following signs or symptoms of dysuria, urinary frequency, urinary urgency, and suprapubic pain (FDA) [36]
- Female patients with documented pyuria (WBC≥10/μL) and a minimum number of symptoms such as frequency, urgency and dysuria (EMA) [37]
In both guidelines, FDA and EMA, for primary clinical outcome only patients with a so-called “significant” bacteriuria with ≥105 colony forming units (CFU)/ml can be considered. However, significant bacteriuria of ≥105 CFU/mL in adults was originally defined as significant only for the diagnosis of pyelonephritis [38]. In 1982, Stamm et al. [39] documented that the levels of ≥105 CFU/mL of a pathogen in urine have a very high specificity (99%) but a very low sensitivity (51%) for the diagnosis of uAC. Bacteriuria of ≥102 CFU/mL was suggested by the authors as the best diagnostic criterion (sensitivity, 95%; specificity, 85%). In 2013, Hooton et al. [40] confirmed that E. coli identified as low as 101–102 CFU/mL was sensitive and specific for the diagnosis of AC in symptomatic women. But still, about 20% of these symptomatic female patients were culture “negative” even when being tested for such low counts. Quantitative PCR (qPCR) for E. coli and S. saprophyticus finally demonstrated that almost all women with symptoms suggestive of UTIs and a “negative” culture still have an infection with E. coli [41].
We aimed to reassess first the diagnostic values of these proposed guidelines using the Acute Cystitis Symptom Score (ACSS), which was originally developed in Uzbek and Russian languages [42] and is now validated in several other languages as well (https://www.acss.world/). The ACSS diagnostic part A (Attachment 1) consists of 6 questions concerning typical symptoms, 5 questions concerning potential differential diagnosis, 3 questions concerning quality of life (QoL) and 5 additional questions about concomitant conditions such as female cycles and diabetes mellitus [43]. Each of the typical and differential symptoms and QoL questions can be answered according to 4 levels of severity (none, mild, moderate, severe) and the additional questions can only be answered with yes or no [43].
In the analysis of the study, a total of 517 evaluable female respondents (285 patients with uAC with ages between 16 and 87 years and 232 controls without uAC with ages between 15 and 73 years) were included having used the ACSS in different languages: Uzbek (393), Tajik (65), German (43), Hungarian (16) [44]. The receiver operating characteristic (ROC) curves for the six individual typical symptoms and the summary score of the six symptoms proposed by ACSS showed that the best balance between sensitivity and specificity for the diagnosis of uAC can be reached if all 6 symptoms are considered together (Figure 1). ROC curve analysis revealed the largest area under the curve (AUC) for the summary score of the “typical” domain of the ACSS (AUC [95% confidence interval (CI)]=0.93 [0.91; 0.95]), followed in descending order by dysuria (0.85 [0.82; 0.88]), urgency (0.85 [0.82; 0.88]), sense of incomplete bladder emptying (0.79 [0.75; 0.83]), suprapubic pain (0.74 [0.70; 0.78]), and visible blood in urine (0.63 [0.60; 0.67]).
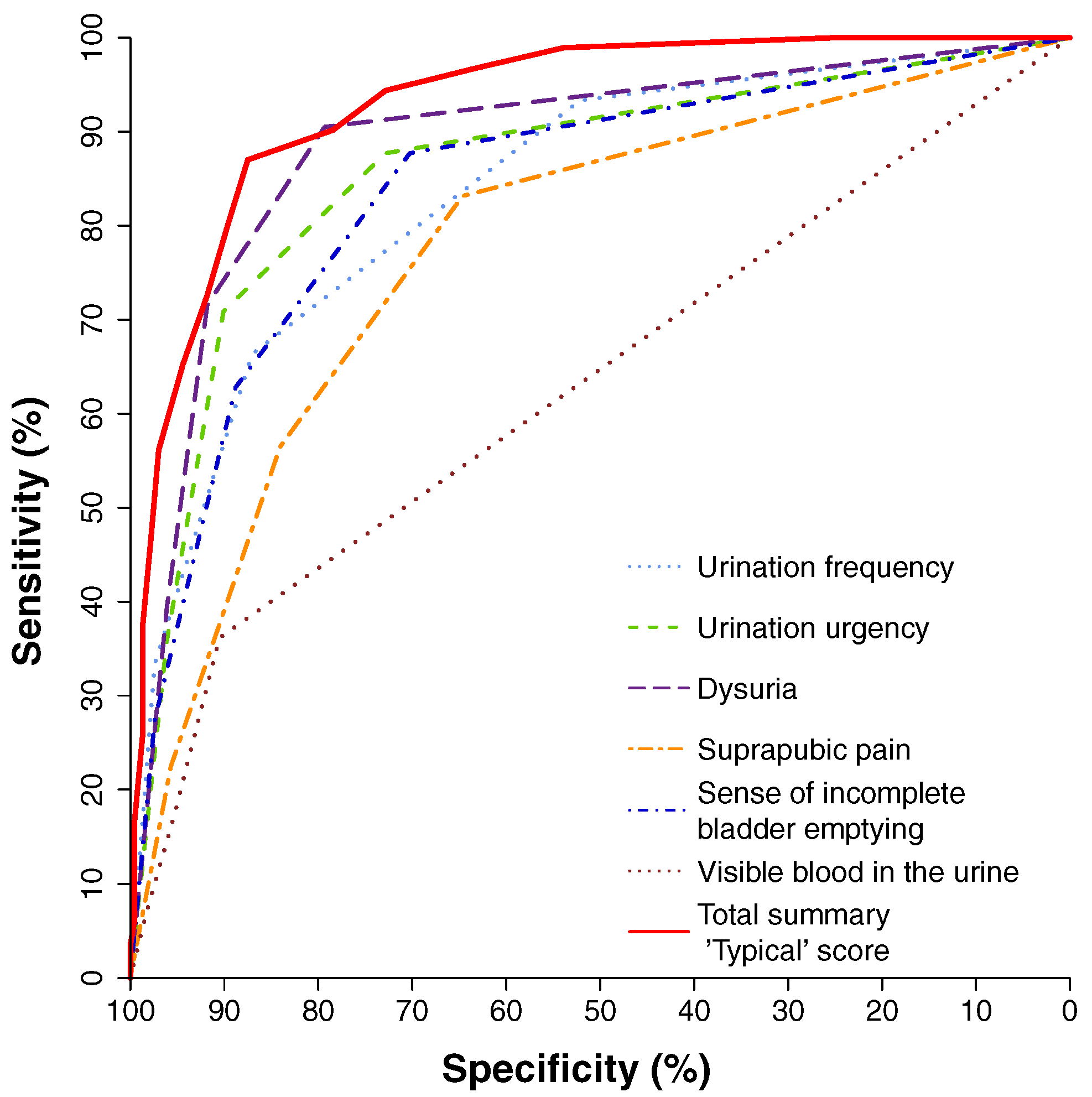
Source: Alidjanov et al. [44], licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/)
Comparing only the 3 symptoms listed in the EMA guidelines with the 4 symptoms mentioned in the FDA guidelines and with the 6 symptoms mentioned in the ACSS, the sensitivity and specificity (average [95% CI]) for these different approaches of the diagnosis of uAC were (a) 0.84 [0.79; 0.88] and 0.83 [0.77; 0.87] for the EMA approach; (b) 0.83 [0.78; 0.87] and 0.88 [0.84; 0.92] for the FDA approach; and (c) 0.87 [0.83; 0.91] and 0.88 [0.83; 0.91] for the ACSS approach using a summary score of 6 and above as the cut-off, respectively (Figure 2).
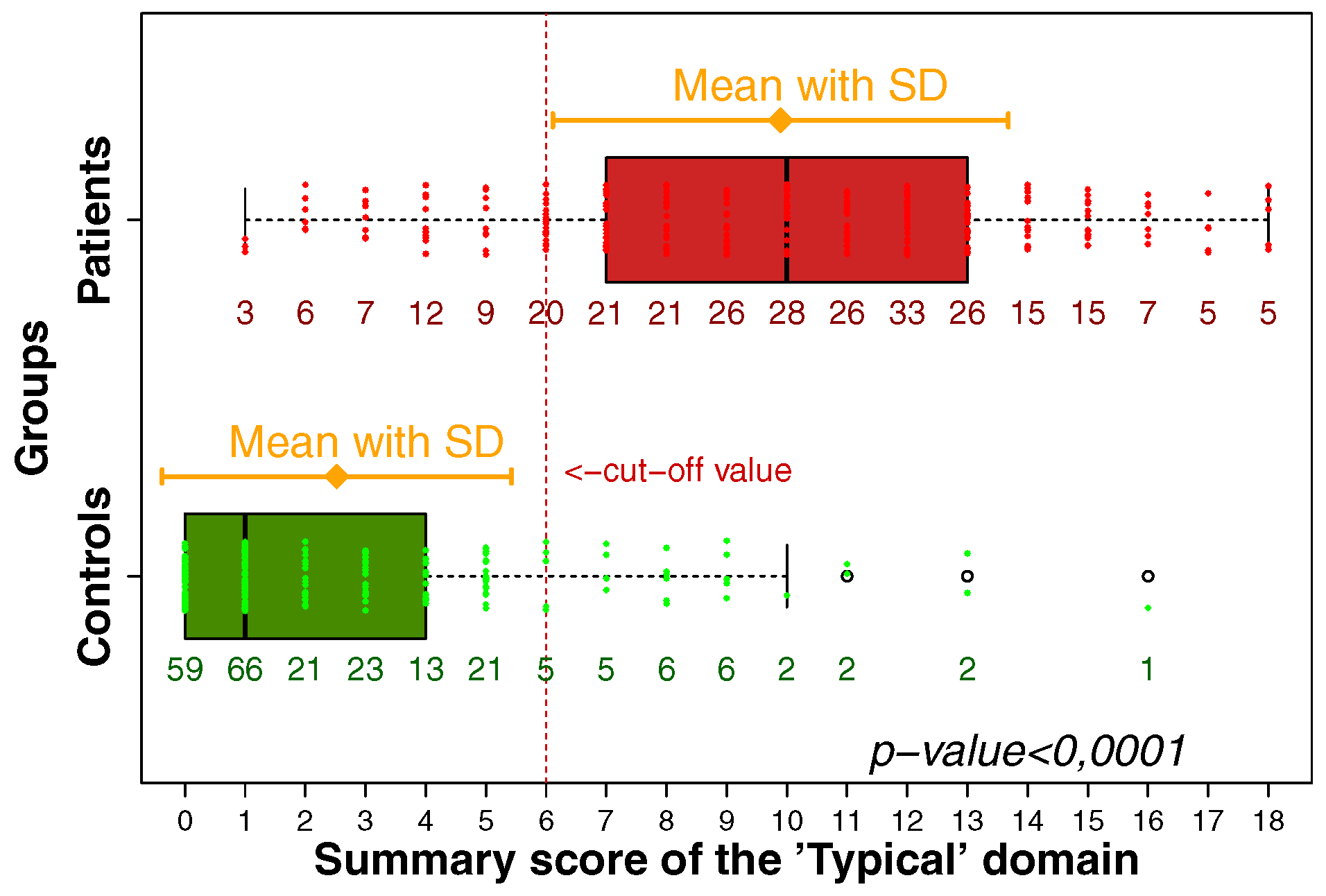
Source: Alidjanov et al. [44], licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/)
Therefore, it is also important to realize that not just the presence of a so-called typical symptom does differentiate significantly between patients with uAC and controls without uAC, but also the severity needs to be considered. Symptoms which are only considered mild do not differ significantly between the two patient groups (Figure 3).
Source: Alidjanov et al. [44], licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/)
Bacterial isolates in female patients with uncomplicated acute cystitis
It is important to consider only those patients for analysis who also have the typical symptoms and the clinical diagnosis of uAC. Because of missing clinical data, laboratories often include all outpatients with UTI into the analysis who may suffer from different categories of UTI. It is also important to consider not only pathogens cultured in urine with CFU ≥105/ml, but also bacteriuria with lower rates of CFU as mentioned earlier.
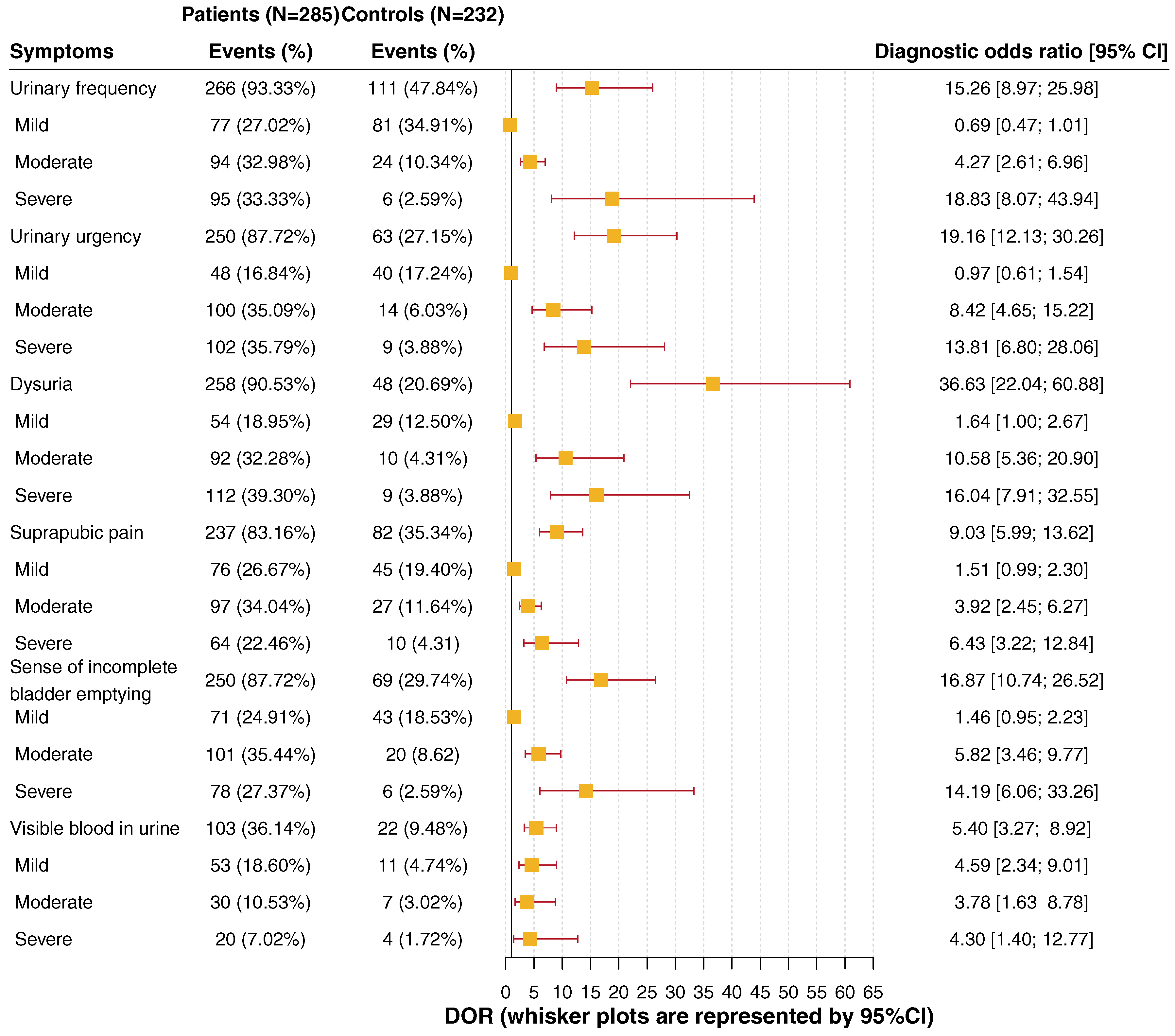
In a single-blind, randomized, multicentre study with 20 urologists in private practice, female patients aged 18 to 75 years with signs and symptoms of uAC were recruited to compare treatment of fosfomycin trometamol with ofloxacin and co-trimoxazole [45]. Midstream or catheter urine was collected for urine culture. The urine was also microscopically investigated for leucocytes and erythrocytes. At first visit of the 421 evaluable patients, 308 patients had a bacteriuria of ≥105 CFU/ml, 79 had 102–104 CFU/ml, 21 had ≤101 CFU/ml, and 13 had no culture performed. The distribution of bacterial isolates cultured in the 387 patients with ≥102 CFU/ml are shown in Table 2, including 360 monoinfections and 27 mixed infections. E. coli was detected in 298 (77.0%) patients with monoinfections and 22 (5.7%) patients with mixed infections: a total of 320 (82.7%) patients (Table 2).
| Monoinfections | CFU >105/ml | CFU 102–104/ml | Total n (%) |
| Escherichia coli | 237 | 61 | 298 (77.0%) |
| Proteus mirabilis | 17 | 1 | 18 (4.7%) |
| Staphylococcus epidermidis | 14 | – | 14 (3.6%) |
| Klebsiella oxytoca | 4 | 2 | 6 (1.6%) |
| Staphylococcus aureus | 2 | 2 | 4 (1.0%) |
| Staphylococcus saprophyticus | 4 | – | 4 (1.0%) |
| Citrobacter diversus | 2 | 2 | 4 (1.0%) |
| Enerococcus faecalis | 3 | 3 | 6 (1.6%) |
| Klebsiella pneumoniae | 3 | – | 3 (0.8%) |
| Pseudomonas aeruginosa | 1 | – | 1 (0.3%) |
| Enterobacter aerogenes | 1 | – | 1 (0.3%) |
| Citrobacter amalonaticus | – | 1 | 1 (0.3%) |
| Mixed infections | |||
| E. coli/P. mirabilis | 8 | 1 | 9 (2.3%) |
| E. coli/K. pneumoniae | 3 | – | 3 (0.8%) |
| E. coli/Acinetobacter spp. | 1 | – | 1 (0.3%) |
| E. coli/E. faecalis | 3 | – | 3 (0.8%) |
| E. coli/Enterococcus spp. | – | 2 | 2 (0.5%) |
| E. coli/S. aureus | 1 | 1 | 2 (0.5%) |
| E. coli/S. epidermidis | 1 | 1 | 2 (0.5%) |
| P. mirabilis/E. faecalis | 1 | 1 | 2 (0.5%) |
| C. amabonaticus/E. faecalis | 1 | – | 1 (0.3%) |
| C. freundii/E. faecalis | 1 | – | 1 (0.3%) |
| S. epidermidis/Streptococcus group B | – | 1 | 1 (0.3%) |
| Patients (n) | 308 | 79 | 387 (100%) |
This and many other studies have shown that E. coli is the most frequent and most important uropathogen for causing uAC. Therefore, according to the German National S3 Guideline, the detection of E. coli in symptomatic women is predictive for a bacterial UTI, irrespective of the number of pathogens. In contrast, the presence of Enterococci and group B Streptococci in urine is not predictive for UTIs and needs further investigations [34].
Pathogenicity properties of uropathogen
Uropathogens such as E. coli must have specific pathogenic properties in order to trigger a clinically manifest infection. Bacterial attachment to the uroepithelium is the necessary initiating event permitting bacterial persistence, and also stimulates early activation of the innate immune system.
Fimbriae/pili and nonfimbrial adhesins are responsible for adherence to the uroepithelium [46]. Type 1 fimbriated (fimH) E. coli strains are the predominant phenotypic variant isolated from patients with uUTI, and the presence of this adhesin is essential for establishing acute cystitis. Some of these adhesins (e.g. type 1 pili) also mediate internalization into the uroepithelium. Nests of intracellular persistent bacteria can be a source of recurrent infections [47].
Toxins such as alpha-hemolysin (α-Hly), cytotoxic necrotizing factor (CNF) or secreted autotransporter toxin (SAT) influence signal transduction mechanisms and modulate the host’s defense behavior. They also induce the death of uroepithelial cells (apoptosis, necrosis). Siderophores such as enterobactin, salmochelin, aerobactin and yersiniabactin bind with high affinity iron, which is an essential cofactor of many enzymes and therefore plays a role in the survival and growth of uropathogenic microorganisms [48].
The expression of these pathogenicity factors is strictly regulated and encoded by genetic determinants. This means that potential infectious agents can, on the one hand, adapt to host-specific defense functions and, on the other hand, trigger an infection if these are disrupted [48]. Local urinary cytokines regulate host defense against UTI. Activation of Toll-like receptors on uroepithelial cells promotes release of cytokines which, in turn, recruit and activate granulocytes, macrophages, monocytes and other immune regulatory cells [49].
3 Antibiotic treatment of uncomplicated acute cystitis
In most guidelines so far antibiotic therapy of the acute episode of uAC is mentioned in first place [1], [11], [12], [13], [15], [34], [50], [51], [52]. Therefore, at best the local antibiotic resistance especially of E. coli, the most frequent and important uropathogen causing uAC, should be known, which may differ between countries and areas.
Real-world data were collected in five different areas of Germany by physicians from three medical specialities (urology, general medicine/internal medicine/primary care, and obstetrics/gynecology) to examine antimicrobial resistance (AMR) prevalence, treatment patterns, and clinical outcomes among female patients with uUTI [53]. Data were collected from a retrospective physician-based chart review completed by physicians treating patients with uUTI. Non-pregnant women aged ≥12 years, with a uUTI diagnosis, an E. coli-positive urine culture between January 2017 and December 2019, and susceptibility test results according to EUCAST breakpoints for at least ≥4 drug classes were eligible, while “susceptible at higher dose/susceptible + intermediate” was used for analysis.
Patients were stratified into three cohorts by E. coli susceptibility to six drug classes (fosfomycin; nitrofurantoin; mecillinam; fluoroquinolones including ciprofloxacin, levofloxacin, and ofloxacin; cefpodoxime; folate metabolism inhibitors (FMIs) including trimethoprim and trimethoprim-sulfamethoxazole): susceptible to all (SUS), resistant to one or two drug classes (DR1/2), and resistant to ≥3 (MDR) drug classes tested. Among 386 eligible patients [SUS (67.1%); DR1/2 (29.0%); MDR (3.9%)], AMR prevalence was FMIs (18.3%) and lowest for fluoroquinolones (5.2%). The drugs prescribed most often were fosfomycin in SUS (44.0%), DR1/2 (41.4%), and fluoroquinolones in MDR (40.0%) (Table 3). Between the 5 regions in Germany, one could observe some differences in the rate of resistance of more than 10% for mecillinam and the fluoroquinolones. The resistance rates for trimethoprim or cotrimoxazole were in the range around 20% (17.5%–26.1%) except in the southwest with only 12.7%, whereas in the northeast and in the southeast the resistance rates were >20% (Table 4).
| Index drug class | All patients (n=386) |
Antimicrobial susceptibility cohort | ||
| SUS (n=259) | DR1/2 (n=112) | MDR (n=15) | ||
| Fosfomycin | ||||
| Tested (n, 100%) | 373 (100) | 248 (100) | 110 (100) | 15 (100) |
| Susceptible* | 353 (94.6) | 248 (100) | 99 (90.0) | 6 (40.0) |
| Resistant | 20 (5.4) | 0 (0.0) | 11 (10.0) | 9 (60.0) |
| Nitrofurantoin | ||||
| Tested (n, 100%) | 368 (100) | 249 (100) | 105 (100) | 14 (100) |
| Susceptible* | 344 (93.5) | 249 (100) | 88 (83.8) | 7 (50.0) |
| Resistant | 24 (6.5) | 0 (0.0) | 17 (16.2) | 7 (50.0) |
| Mecillinam | ||||
| Tested (n, 100%) | 364 (100) | 242 (100) | 107 (100) | 15 (100) |
| Susceptible* | 319 (87.6) | 242 (100) | 72 (67.3) | 5 (33.3) |
| Resistant | 45 (12.4) | 0 (0.0) | 35 (32.7) | 10 (66.7) |
| Fluoroquinolones | ||||
| Tested (n, 100%) | 345 (100) | 228 (100) | 103 (100) | 14 (100) |
| Susceptible* | 327 (94.8) | 228 (100) | 91 (88.3) | 8 (57.1) |
| Resistant | 18 (5.2) | 0 (0.0) | 12 (11.7) | 6 (42.9) |
| Cefpodoxime | ||||
| Tested (n, 100%) | 347 (100) | 230 (100) | 103 (100) | 14 (100) |
| Susceptible* | 314 (90.5) | 230 (100) | 76 (73.8) | 8 (57.1) |
| Resistant | 33 (9.5) | 0 (0.0) | 27 (26.2) | 6 (42.9) |
| FMI (TMP, SXT) | ||||
| Tested (n, 100%) | 268 (100) | 178 (100) | 78 (100) | 12 (100) |
| Susceptible* | 219 (81.7) | 178 (100) | 37 (47.4) | 4 (33.3) |
| Resistant | 49 (18.3) | 0 (0.0) | 41 (52.6) | 8 (66.7) |
| * susceptible+intermediate/susceptible at higher dose (breakpoints according to EUCAST) | ||||
| Index drug class | All patients (n=386) |
Region in Germany | ||||
| Northeast (n=38) |
Northwest (n=57) |
Southeast (n=90) |
Southwest (n=119) |
West (n=82) |
||
| Fosfomycin | ||||||
| Tested (n, 100%) | 373 (100) | 37 (100) | 56 (100) | 88 (100) | 113 (100) | 79 (100) |
| Susceptible* | 353 (94.6) | 36 (97.3) | 54 (96.4) | 85 (96.6) | 105 (92.9) | 73 (92.4) |
| Resistant | 20 (5.4) | 1 (2.7) | 2 (3.6) | 3 (3.4) | 8 (7.1) | 6 (7.6) |
| Nitrofurantoin | ||||||
| Tested (n, 100%) | 368 (100) | 37 (100) | 57 (100) | 82 (100) | 112 (100) | 80 (100) |
| Susceptible* | 344 (93.5) | 37 (100) | 56 (98.2) | 77 (93.9) | 101 (90.2) | 73 (91.3) |
| Resistant | 24 (6.5) | 0 (0.0) | 1 (1.8) | 5 (6.1) | 11 (9.8) | 7 (8.8) |
| Mecillinam | ||||||
| Tested (n, 100%) | 364 (100) | 35 (100) | 57 (100) | 83 (100) | 117 (100) | 72 (100) |
| Susceptible* | 319 (87.6) | 25 (71.4) | 52 (91.2) | 76 (91.6) | 102 (87.2) | 64 (88.9) |
| Resistant | 45 (12.4) | 10 (28.6) | 5 (8.8) | 7 (8.4) | 15 (12.8) | 8 (11.1) |
| Fluoroquinolones | ||||||
| Tested (n, 100%) | 345 (100) | 36 (100) | 50 (100) | 83 (100) | 117 (100) | 70 (100) |
| Susceptible* | 327 (94.8) | 31 (86.1) | 45 (90.0) | 74 (93.7) | 107 (97.3) | 70 (100) |
| Resistant | 18 (5.2) | 5 (13.9) | 5 (10.0) | 5 (6.3) | 3 (2.7) | 0 (0.0) |
| Cefpodoxime | ||||||
| Tested (n, 100%) | 347 (100) | 34 (100) | 50 (100) | 84 (100) | 106 (100) | 73 (100) |
| Susceptible* | 314 (90.5) | 30 (88.2) | 46 (92.0) | 78 (92.9) | 93 (87.7) | 67 (91.8) |
| Resistant | 33 (9.5) | 4 (11.8) | 4 (8.0) | 6 (7.1) | 13 (12.3) | 6 (8.2) |
| FMI (TMP, SXT) | ||||||
| Tested (n, 100%) | 268 (100) | 23 (100) | 46 (100) | 71 (100) | 71 (100) | 57 (100) |
| Susceptible* | 219 (81.7) | 17 (73.9) | 37 (80.4) | 56 (78.9) | 62 (87.3) | 47 (82.5) |
| Resistant | 49 (18.3) | 6 (26.1) | 9 (19.6) | 15 (21.1) | 9 (12.7) | 10 (17.5) |
| * susceptible+intermediate/susceptible at higher dose (breakpoints according to EUCAST) | ||||||
Treatment for uUTI failed for 8.8% of patients; failure was more likely in MDR versus SUS [adjusted odds ratio [95% CI]=4.21 [1.14–1.50]; P=0.031); incidence of recurrent infection in the 6-months post-index period was higher in DR1/2 versus SUS. These findings may have implications for empiric prescribing, suggesting an unmet need for new treatments [53].
In the updated EAU guidelines 2024, the first line oral antibiotics recommended for treatment of uAC in women are fosfomycin trometamol, nitrofurantoin, pivmecillinam, alternatively oral cephalosporins, e.g. during pregnancy. Trimethoprim or cotrimoxazole are only recommended if the local resistance pattern for E. coli is <20%, which e.g. in Germany is not anymore the case everywhere [1]. The German guidelines [54] also recommend in addition nitroxoline as a first line oral antibiotic for treatment of uAC (Table 5), which is available in 9 European countries (Bulgaria, Croatia, Germany, Georgia, Lithuania, Poland, Romania, Russia and Serbia). In a phase 3 study, the efficacy of nitroxoline was non-inferior with cotrimoxazole and ofloxacin (95% confidence interval <10%) and a recent microbiological study in Germany showed that E. coli from uAC were 100% susceptible to nitroxoline [55], [56].
| Substance | Daily dose | Duration (days) |
| The following antibiotics should be used preferentially in the treatment of UC | ||
| Fosfomycin-trometamol | 3,000 mg 1x daily | 1 |
| Nitrofurantoin | 50 mg 4 x daily | 7 |
| Nitrofurantoin RT (slow-release form) | 100 mg 2x daily | 5 |
| Nitroxolin | 250 mg 3x daily | 5 |
| Pivmecillinam | 400 mg 2–3x daily | 3 |
| Trimethoprim should not be used of first choice if local resistance to E. coli is >20% | ||
| Trimethoprim | 200 mg 2x daily | 3 |
| The following antibiotics should not be used as drug of first choice in the treatment of UC | ||
| Cefpodoxim-proxetil | 100 mg 2x daily | 3 |
| Ciprofloxacin | 250 mg 2x daily | 3 |
| Cotrimoxazole | 160/800 mg 2x daily | 3 |
| Levofloxacin | 250 mg 1x daily | 3 |
| Norfloxacin | 400 mg 2x daily | 3 |
| Ofloxacin | 200 mg 2x daily | 3 |
4 Alternative management, non-antibiotic therapy of uncomplicated acute cystitis in women
Already in 2012, the American infectiologist T.M. Hooton rightly assessed antibiotic prescriptions for uUTI very critically [57]. Around 25% of all antibiotic prescriptions are for uUTI, although many such conditions are self-limiting or could be treated non-antibiotically and therefore antibiotics are not always necessary. This approach limits the development of resistance, reduces drug costs, influences the risk of collateral damage and improves the compliance of affected individuals.
A symptom-oriented treatment with non-steroidal analgesics/anti-inflammatory drugs has been investigated in several studies. This showed a significant reduction in the use of antibiotics [58], [59], [60]. However, with this strategy the symptoms lasted slightly longer and slightly more cases of pyelonephritis were observed, although the recurrence rate was not increased after symptomatic therapy as compared to antibiotic therapy [61], [62], [63].
The treatment of uAC with herbal preparations, for example with BNO 1045 (Canephron: lovage root, rosemary leaves, centaury herb), showed non-inferiority in terms of symptom duration compared to antibiotic therapy with fosfomycin [64]. This study also showed that the local immune reaction due to the infection could be normalized through therapy with this phytotherapeutic agent [65]. Therefore, non-antibiotic therapeutic measures should be considered as a treatment option for uUTIs which should be discussed with the patient [54].
Patient-reported outcome
In clinical studies with non-antibiotic therapy, but also in so called non-interventional studies (NIS), in which usually no urine cultures are performed, the elimination of bacteriuria cannot be the therapeutic aim. Therefore, the clinical outcome is much more important, for which we need standardized measures. The 2nd part of the ACSS is suitable as a patient-reported outcome measure (PROM), which was developed in this international study mentioned earlier as part 2 [44], [66]. 134 patients of among 517 previously selected female respondents [44] were included into the patient-reported outcome (PRO) analysis. The age of the selected patients ranged from 17 to 82 years, with a median (IQR) of 31 (24.00–44.25) and mean (SD) of 36.28 (16.03) years. Of these, 109 filled out at least 1 copy of the “follow-up Part B” form of the ACSS (Attachment 1) (one “follow-up” visit) after the initial “diagnostic” visit and 25 patients filled out multiple copies at different “follow-up” visits. Altogether, they have formed 236 cases.
Figure 4 shows the reduction of the summary scores of the six typical symptoms (“Typical” domain) of the ACSS at diagnostics of uAC in women (baseline) and at the four different follow-up visit categories. At the visits “end of treatment” and “test of cure”, most of the women had a summary score of the typical symptoms <6. From further analysis a summary score of the “Typical” domain up to a maximum of 5 with no symptom >1 (mild) AND no visible blood in the urine was recommended as threshold for successful clinical outcome for further studies. Using this threshold the treatment was clinically successful at end of treatment in 80.5% and at test of cure in 80.4%. As secondary outcome a summary score of the three Quality of Life (QoL) categories up to 3 with no item >1 and a score of no more than 1 for the overall outcome (Dynamics) listed on the head of the ACSS part B (Attachment 1) should be considered [66].
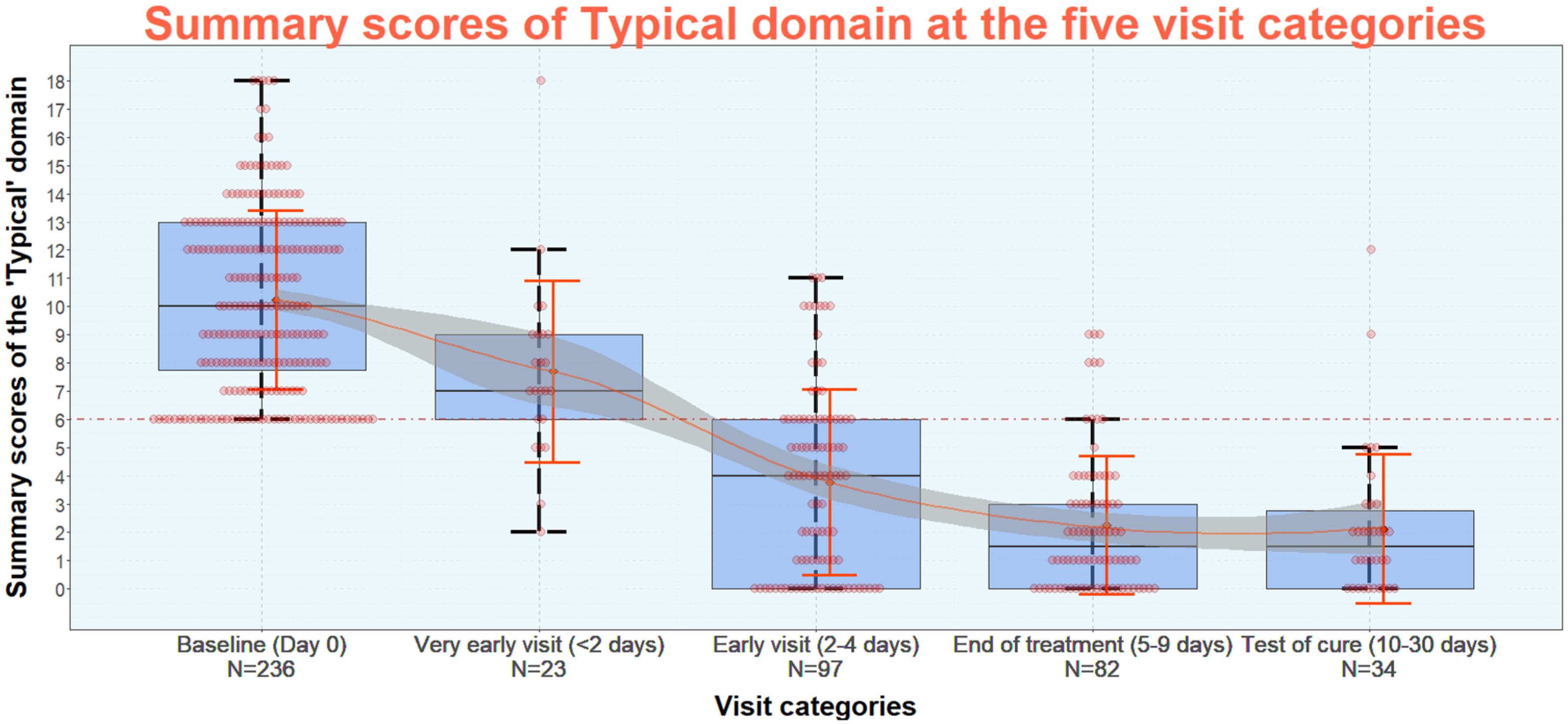
Source: Alidjanov et al. [66], licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/)
Although reports of patients concerning symptoms can only be subjective by definition, by answering the same, in the meantime familiar questionnaire at any follow-up visit, one can at least expect that by scoring the symptoms not only the presence or absence, but also the increasing or decreasing severity of each symptom reported by the patient can be considered as a quasi-objective measure.
Since non-antibiotic therapy has become an alternative approach to treat uAC in women, suitable PROMs are urgently needed. Although typical symptoms are mainly used for clinical diagnosis and outcome, these symptoms are not exclusively found in uAC. Therefore, severity scoring of the symptoms is needed not only for diagnostics, but also for PROM to define “clinical cure” of any intervention. The presented data analysis demonstrated that the ACSS has the potential to be used as a suitable instrument for PROM in well-designed prospective clinical studies [66].
A systematic literature review revealed 23 studies reporting on six different PROMs for uUTI in women. From those, the Acute Cystitis Symptom Score (ACSS) and the Urinary Tract Infection-Symptom and Impairment Questionnaire (UTI-SIQ-8) were recommended for further use. Both instruments showed sufficient content validity [67].
5 Recurrent uncomplicated cystitis in women
The current European Association of Urology (EAU) guidelines define recurrent urinary tract infections (UTIs) as recurrences with a frequency of at least three UTIs in the past year, or two UTIs in the last 6 months [1]. Risk factors for recurrent UTI are discussed in depth by Cai [68]. The principal risk factor in sexually active pre-menopausal women is frequency of sex [68], [69]. Other behaviors including use of spermicide, having a new sexual partner within the past year, pre-/post-coital voiding habits, delayed voiding habits/periodicity of urination and vaginal douching also affect risk of recurrence [68], [69]. In addition, early onset (<15 years old), family history, body-mass index and urine voiding disorders all increase risk in younger women [68], [69]. Major risk factors in older women appear to be substantially related to the effects of reduced estrogen levels and include atrophic vaginitis, cystocele, increased post-void urine volume and functional status deterioration [70], [71]. Cai et al. have analysed the risk of UTI recurrence for various factors and created a nomogram for the calculation of the overall risk for UTI recurrence which has substantial clinical utility (Table 6) [72].
| Categories | Univariate analysis (P) | Multivariate analysis (P) |
| HR (95% CI) | HR (95% CI) | |
| Age | P 0.76; HR 0.81 (0.17–1.23) | P 0.76; HR 0.81 (0.17–1.23) |
| Marital status | P 0.26; HR 0.72 (0.15–1.43) | P 0.65; HR 1.01 (0.54–1.41) |
| Sexual encounters per week | P 0.09; HR 1.22 (0.67–1.98) | P 0.07; HR 1.10 (0.64–1.77) |
| No. partners | P 0.01; HR 3.06 (2.00–3.99) | P 0.003; HR 2.97 (1.50–3.67) |
| Contraceptive use | P 0.02; HR 1.80 (1.16–2.21) | P 0.12; HR 1.61 (0.87–1.79) |
| Hormonal status | P 0.03; HR 4.52 (3.10–5.65) | P 0.001; HR 5.97 (4.11–6.51) |
| Smoking | P 0.08; HR 0.81 (0.14–1.33) | P 0.76; HR 0.90 (0.34–1.45) |
| Alcohol use | P 0.56, HR 1.04 (0.50–1.75) | P 0.07; HR 1.32 (0.80–1.83) |
| Parity | P 0.01; HR 0.91 (0.17–1.94) | P 0.09; HR 0.7 (0.07–1.01) |
| No. UTI per year | P 0.03; HR 2.16 (1.98–2.77) | P 0.003; HR 3.17 (2.54–3.88) |
| Bowel function | P 0.02; HR 2.96 (2.11–4.07) | P 0.001, HR 3.44 (2.81–5.89) |
| Type of pathogens isolated – Gram-positive/negative |
P 0.01; HR 3.11 (2.32–4.78) | P 0.001; HR 3.91 (2.66–4.35) |
| Water intake | P 0.03; HR 2.12 (1.87–3.02) | P 0.11; HR 2.22 (1.57–3.09) |
| Previous treatment of ASB | P 0.03; HR 4.96 (3.54–6.69) | P 0.001; HR 5.44 (3.51–7.81) |
| Response to oral antibiotic therapy | P 0.55; HR 0.61 (0.25–1.08) | P 0.87; HR 0.99 (0.21–1.44) |
| ASB – asymptomatic bacteriuria. P-values were calculated using two-sided log-rank test; P<0.05 (significant); HR – hazard ratio; CI – confidence interval | ||
The relationship between acute symptomatic UTI and reduced quality of life (QoL) has been well established for some years [72], [73]. Naber et al. [74] performed a PubMed/MEDLINE search on literature from 2000 to 2020 using the terms (“recurrent UTI” OR “recurrent urinary tract infection”) AND (“anxiety” OR “depression” OR “quality of life”) in order to identify any recent high-quality meta-analyses or systematic reviews as well as other relevant publications and found ten studies appropriate for their review.
In one such study using data from a large UK Internet self-help forum hosted by a charity supporting people with bladder problems (N=5,994), the authors highlighted patient descriptions of reduced quality of both intimate and social relationships, self-esteem, and capacity for work due to recurrent UTI [75]. Women also frequently described broader systemic disabling symptoms than those typically ascribed to UTI, including flu-like symptoms, spasms, and both back and leg pain [75]. In addition, seemingly mild symptoms such as increased frequency and urgency of urination were discussed in terms of their anxiety-inducing effects and disruption to sleep patterns with the potential to cause persistent fatigue [75]. One contributor stated: “I find that this affects every aspect of my life”.
The European GESPRIT (GErmany, Switzerland, Poland, Russia and ITaly) study used a self-administered online survey which assessed course of disease; social and economic burden; disease management and QoL effects (SF-12v2 questionnaire) related to recurrent UTI [29]. The study included adult women who had suffered from recurrent UTI and who were currently affected by an acute UTI or had experienced an episode within the 4 weeks prior to entering the study. Approximately three days of sick leave were taken per year across the full study population (2.3 days in Switzerland to 3.9 days in Germany). Limitations to daily activity occurred on approximately 3.5 days per year in the full population (2.6 in Poland to 4.0 days in Russia) [30]. The mean number of doctor visits per year was 2.8, ranging from 1.7 visits in Russia to 3.7 visits in Germany (P<0.0001).
For the mental components of SF-12v2, the most significant reduction overall was in mental role functioning, followed by the previously mentioned social functioning and mental health, which both reduced by a similar extent [29]. With the exception of vitality, all mental-health components were reduced by a similar or greater extent than physical components, and the overall mental score was substantially lower. Of the 90% of women who answered questions related to sexual function (n=1,745), 34% suffered from UTI very often or often after sexual intercourse, with a substantially higher proportion of patients (57%) stating that sexual relations were negatively influenced by UTI [29].
Specific aspects of microbiology in recurrent uncomplicated cystitis
In female patients with recurrent uAC, the distribution of uropathogens is about the same as for patients with sporadic cystitis during the acute episode (Table 2) [45]. Asymptomatic bacteriuria (ABU) between the acute episodes, however, has shown not to be an additional risk factor for recurrent UTI. In contrast, ABU may even reduce the number of recurrences as has been shown [76]. Therefore, ABU should also not be treated in patients with recurrent UTI.
In another study, 20 patients were randomized to blinded inoculations with E. coli 83972 or saline into the bladder [77]. In phase 1, the time to the first UTI was longer with than without E. coli 83972 bacteriuria (median 11.3 vs 5.7 months, sign test p=0.0129). Phase 2 was analyzed after patients had spent a total of 202 months with and 168 months without E. coli 83972 bacteriuria. There were fewer reported UTI episodes with vs without E. coli 83972 bacteriuria (13 vs 35 episodes, paired t test p=0.009, CI 0.31–1.89). There was no febrile UTI episode in either of the study arms and no significant side effects of intravesical bacterial inoculation were reported [77].
On the other hand, the uropathogens causing recurrent episodes of UTI may also stay in the bladder environment between the episodes. Naboka et al. [78], [79] investigated midstream urine samples taken from 169 women between episodes of recurrent lower UTI (LUTI) and analyzed the strains for virulence factor genes (VFGs) often grouped into clusters called “pathogenicity islands” because the pathogenic potential of microorganisms depends on the presence and/or appearance of their specific properties to interact with the host. Sixty-two strains of Enterobacteriaceae at concentrations 102–108 CFU/ml were analyzed for the presence of the following VFGs:
Adhesive structures coding
P-pili group:
- papA is the structural subunit of P pili (colonization factor in extraintestinal infections)
- papE/F – apical adhesion of P pili
- papGII – apical adhesion of P pili (II allele)
- afa – afimbrial adhesin (adhesin binds to the DAF receptor on the epithelium of the cell surface, also provides the ability to hemagglutination)
- bmaE – M pili
Coding of iron absorption systems:
- fyuA, iutA, feoB – synthesis of siderophores
General pathogenicity:
- kpsMII – capsule synthesis
- usp – uropathogenic specific protein
In all strains VFGs were found with numbers from 1 to 10. Four VFGs were found at all levels of bacteriuria (from 102 to 108) in most strains (>50%): papGII, feoB, fyuA and usp. Each of the genes papA, papE/F and usp was found more often in uropathogens from patients with a higher level of leukocyturia. The authors concluded that the inter-episode period in recurrent LUTI is associated with varying levels of bacteriuria of enterobacteria. Since enterobacteria virulent potential could be determined at all levels of bacteriuria, there is a potential risk for recurrence of LUTI at all levels of bacteriuria.
Prophylaxis of recurrent uncomplicated cystitis in women
According to the German guidelines, antibiotics should not be used primarily for the prophylaxis of recurrent uUTIs [54]. For prophylaxis, sufficient drinking quantities are sometimes sufficient, which should be at least 1.5 liters per day [80]. Regarding the value of cranberries in preventing recurrent UTIs, Jepson et al. found in their Cochrane review 2012 that cranberry products did not significantly reduce the incidence of symptomatic UTI overall or in women with recurrent UTI compared to placebo, water or no treatment [81]. However, recent systematic reviews including meta-analyses were able to prove that consumption of mono- or combination products containing cranberries had indeed a positive preventive effect on the rate of UTI recurrences in otherwise healthy women [82], [83], [84], [85], [86], [87]. This result was also confirmed in the most recent Cochrane review 2023 [88].
The different study results may be due to the clinical and methodological heterogeneity of the included studies. One explanation for this would be, for example, the different content of proanthocyanidin (PAC) in the different products. Cranberry has been shown to inhibit the adhesion of uropathogenic E. coli to uroepithelial cells, which is caused by PAC in vitro in a dose-dependent relationship [89], [90], [91]. Since the data currently available for comparison with long-term antibiotic prevention are contradictory, according to the EAU guidelines there is only a weak recommendation to use cranberry to prevent recurrent UTIs [1]. Other phytotherapeutics should also be discussed, e.g. mustard oils from watercress and horseradish [92].
The use of D-mannose to treat uUTIs is based on insights into the process of bacterial cytoadherence. D-mannose has structural similarities to those glycoproteins. Uroepithelium (uroplakin) binds to the E. coli via their type 1 fimbriae. This means that D-mannose can competitively inhibit the adhesion of infectious pathogens in the urinary tract [93]. A prospective, randomized, clinical study has shown that this strategy may be an alternative to the use of antibiotics in women with uncomplicated recurrent UTI [94].
Two systematic reviews including a meta-analysis examined the effect of D-mannose on the rate of recurrent UTI. Lenger et al. analyzed data from 390 patients and came to the conclusion that D-mannose is effective in preventing recurrent urinary tract infections compared to placebo and has comparable effectiveness to antibiotic prophylaxis [95]. D-mannose was well tolerated, with only 8/103 (7.8%) patients complaining of diarrhea. In another systematic review with 695 patients, D-mannose was shown to improve quality of life, significantly reduce recurrent urinary tract infections in both catheter users and non-catheterized patients, reduce the incidence of rUTIs and prolong the recurrence-free time [96]. However, a current systematic Cochrane review, in which a total of 719 patient data were analyzed, was unable to determine whether D-mannose significantly reduces the number of recurrent urinary tract infections compared to no treatment, other dietary supplements or antibiotics [97].
Immunomodulation is another option for prevention of recurrent UTI. The goal is to activate specific immunity to improve humoral and cell-mediated innate defense mechanisms in the host organism. The first available agent, OM-89, is administered orally for 3 months and boostered in the 7th to 9th month for 10 days each month. It contains lyophilized bacterial lysates from 18 uropathogenic E. coli strains. In vitro it was effective in stimulating the metabolism of murine spleen cells within a concentration range of 0.625–2.5 mg/ml. The activation of murine bone marrow-derived macrophages by OM-89 was shown by the induction of NO production. In the human system, the effect of OM-89 was tested in vitro: metabolic activity of peripheral blood lymphocytes (PBL) was stimulated starting at concentrations of approx. 250 microg/ml, and the spontaneous apoptosis of polymorphonuclear neutrophils (PMN) was reduced starting at OM-89 concentrations of approx. 100 microg/ml [98]. In three meta-analyses of the five prospective, randomized, placebo-controlled clinical studies between 1990 and 2005 including about 1,000 adult patients with an observation period of 6 (four studies) to 12 (one study) months, the number of UTIs was significantly lower in OM-89-treated patients (mean 39%), as was the use of antibacterials [82], [83], [99], [100].
Urovac is another such possibility which is available as vaginal suppository. It consists of 10 strains of heat-killed uropathogens: six from E. coli of different serotypes and one each from Klebsiella pneumoniae, Proteus vulgaris, Morganella morganii and Enterococcus faecalis. In the four meta-analyses mentioned earlier [82], [83], [99], [100] three randomized clinical trials (RCT) could be analyzed. Primary immunization consisted of 3 vaginal vaccine suppositories at weekly intervals (with 1 [low dose] or 2 [high dose] ampules of 2x109 heat-killed organisms per suppository). In 2 of the 3 studies the addition of booster vaccination was evaluated. Booster immunization consisted of 3 additional vaccine suppositories (with 1x109 killed organisms or 2x109 killed organisms) at monthly intervals. The time until first reinfection, the proportion of women experiencing UTI and the mean number of UTIs during follow-up were all in favor of the booster immunization group compared to those receiving placebo or primary immunization only. The comparison of all the studies above was limited by the nature of definition of UTIs, and the duration over which the interventions were assessed against placebo.
A further option could be the intramuscular StroVac consisting also of 10 strains of heat-killed uropathogens: six from E. coli of different serotypes and one each from Klebsiella pneumoniae, Proteus mirabilis, Morganella morganii and Enterococcus faecalis. It should be injected three times at weekly intervals and boostered with one injection 12 months later. Its efficacy of prophylaxis for recurrent UTI was determined in a two-year follow-up study in women with recurrent UTI with StroVac (n=124) compared to Nitrofurantoin (n=49) 100 mg once daily over three months. In the first 12 months, 86.8% of patients in the StroVac group and 91.8% in Nitrofurantoin group were successful (p=0.22). Side effects were noted in 2.3% in the StroVac group causing discontinuation of therapy, whereas in the Nitrofurantoin group 18.4% stopped medication premature, mostly due to mild diarrhoea. In the second year, 79.3% of patients in the StroVac group were still successful, most of them had undergone booster injection. In contrast, in the Nitrofurantoin group only 59.2% of patients were still successful (p=0.03). Successful vaccination was defined as one or fewer UTIs in 12 months following vaccination and booster injection, respectively [101].
In a recent placebo controlled randomized study, however, 376 patients were randomized. In the StroVac group (n=188), the number of UTIs was reduced from 5.5 to 1.2, in the placebo group (n=188) from 5.4 to 1.3 (p=0.63). StroVac reduced the number of clinically relevant UTIs similar as placebo and did not show statistically significant better results than the placebo [102].
MV140, the most recent option administered as a sublingual spray, is a glycerinated suspension of whole-cell, heat-inactivated bacteria, including equal amounts of four common UTI-causing pathogens: Escherichia coli; Klebsiella pneumoniae; Proteus vulgaris; and Enterococcus faecalis (MV140 formulation) [103]. Since MV140 has shown excellent long-term effectiveness in previous observational studies after 3-month daily administration [103], in a multicenter, randomized, double-blind, placebo-controlled, parallel group 1-year trial, women with recurrent UTI were allocated to receive MV140 for 3 or 6 months or placebo for 6 months in a 1:1:1 ratio. The median (interquartile range) of UTI episodes was 3.0 (0.5 to 6.0) for placebo compared with 0.0 (0.0 to 1.0) in both groups receiving MV140 (P<0.001). Among women treated with placebo, 25% (95% confidence interval [CI], 15% to 35%) were free of UTIs compared with 56% (95% CI, 44% to 67%) and 58% (95% CI, 44% to 67%) of women who received 3 and 6 months of MV140 treatment, respectively [104].
For postmenopausal women with recurrent UTI, topically applied intravaginal estriol cream has shown good results in a randomized, double-blind, placebo-controlled trial [71]. The incidence of UTI in the group given estriol was significantly reduced and more of the women in the estriol group than in the placebo group remained free of UTI during the observational period of 8 months. Lactobacilli were absent in all vaginal cultures before treatment and reappeared after one month in 61% estriol-treated women but in none of the placebo recipients (P<0.001). With estriol the mean vaginal pH declined from 5.5 to 3.8 (P<0.001), whereas there was no significant change with placebo. The authors suggested that the intravaginal administration of estriol prevents recurrent UTI in postmenopausal women, probably by modifying the vaginal flora [71]. Although vaginal estrogens reduced the number of UTIs, oral estrogens did not. In addition, oral estrogens are associated with coronary heart disease, venous thromboembolism, stroke, and breast cancer. Therefore, oral estrogens are not recommended in postmenopausal women to prevent recurrent UTIs [82], [83].
Another option is the use of lactobacilli preparations because specific lactobacilli strains seem to have the ability to interfere with the adherence, growth, and colonization of uropathogenic bacteria [105]. In a double-blind placebo-controlled trial of a Lactobacillus crispatus intravaginal suppository probiotic (Lactin-V; Osel) for prevention of recurrent UTI in premenopausal women high-level vaginal colonization with L. crispatus (≥106 16S RNA gene copies per swab) throughout follow-up was associated with a significant reduction in recurrent UTI only for Lactin-V receiving women (P<0.01) [106]. In other studies, different lactobacilli and different preparations were also used with ambiguous success, e.g. vaginal suppositories containing L. casei v rhamnosus [107] or drinks containing Lactobacillus GG [108] or capsules containing L. rhamnosus GR-1 and L. reuteri RC-14 [109].
L-methionine is a sulfur donor necessary for the biosynthesis of cysteine, a sulfide amino acid. The action of L-methionine achieves a physiological pH value that creates unfavorable conditions for bacterial colonization, since a large number of Gram-negative bacteria are able to alkalize the urine through enzymatic breakdown of urea [110]. Cai et al. evaluated the efficacy of a phytotherapeutic combination of L-Methionine associated with Hibiscus sabdariffa and Boswellia serrata for treatment of acute episodes of uncomplicated UTI in women affected by recurrent UTIs in comparison to antibiotic treatment and demonstrated that this phytotherapeutic combination was able, in comparison to antibiotic treatment, to improve patients’ quality of life, reducing symptoms in acute setting and preventing the recurrences. A significantly higher proportion of patients in the phytotherapy group had asymptomatic bacteriuria (ASB) after three months [110]. This may be of interest because this group also showed that ASB should not be treated in young women affected by UTI because it may play a protective role in preventing symptomatic recurrence [76]. These data have been confirmed by a systematic review and meta-analysis, published in 2021, demonstrating that medical device containing xyloglucan, hibiscus and propolis is superior to comparator regimens in terms of clinical effectiveness in adult women with microbiologically confirmed or clinical suspicion of uncomplicated cystitis and is associated with a high patient compliance [111].
Pentosan polysulfate sodium (PPS) is an oral medication approved by the U.S. Food and Drug Administration for the treatment of painful bladder syndrome/interstitial cystitis to manage pain, urgency, and frequency of urination [112]. Similar to heparin, PPS is a semi-synthetic polysaccharide which has anticoagulant effects. After being excreted to urine, PPS provides a protective coating to the damaged urothelium, restoring the integrity of GAG layer, and decreases the permeability of the urothelium [113]. Tseng et al. [114] conducted a prospective multicenter randomized open-label trial to investigate the efficacy and safety of PPS for prevention of recurrent UTI in women and could demonstrate that a 16-week PPS monotherapy significantly reduced UTI recurrence when compared with an observational group as control.
In a recent multicentre, open label, randomised, non-inferiority trial [115] for prophylaxis of recurrent UTI, methamine hippurate was compared with antibiotic prophylaxis. All participants were observed for at least six months. The modified intention-to-treat analysis comprised 205 (85%) participants (antibiotics, n=102 (85%); methenamine hippurate, n=103 (86%)). Incidence of antibiotic-treated urinary tract infections during the 12 month treatment period was 0.89 episodes per person year (95% confidence interval 0.65 to 1.12) in the anti¬biotics group and 1.38 (1.05 to 1.72) in the methenamine hippurate group, with an absolute difference of 0.49 (90% confidence interval 0.15 to 0.84) confirming non-inferiority. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the methenamine group and most reactions were mild. Therefore, the recent EAU guidelines [1] made a strong recommendation for methemanine hippurate.
According to the EAU guidelines [1], use of endovesical instillations of hyaluronic acid or combinations of hyaluronic acid and chondroitin sulphate to prevent recurrent UTI has only a weak recommendation. Patients should be informed that further studies are needed to confirm the results of initial studies [116], [117], [118].
6 Conclusions
Since uAC is a very frequent infection in women involving several medical specialities, like urology, gynecology, and general medicine, it is important to develop common therapeutic strategies feasible and acceptable not only for daily practice, but also for clinical studies. Although antibiotic therapy of the acute episode and prophylaxis of recurrent UTI may still be an important pillar to be considered as a last resort, non-antibiotic therapy and prophylaxis should be propagated and investigated much further to lower antibiotic usage in general to reduce not only possible adverse events, but in particular selection of antibiotic resistant uropathogens which may be able to cause severe pyelonephritis or even urosepsis in some cases, for which effective antibiotic therapy is absolutely necessary.
Note
This chapter was first published in GMS Infectious Diseases [119].
Dedication
This publication is dedicated to Professor Karl-Horst Bichler for his 90th birthday.
Copyright of the ACSS
The ACSS is copyrighted by the Certificate of Deposit of Intellectual Property in Fundamental Library of Academy of Sciences of the Republic of Uzbekistan, Tashkent (Registration number 2463; 26 August 2015) and the Certificate of the International Online Copyright Office, European Depository, Berlin, Germany (Nr. EU-01-000764; 21 October 2015). The rightsholders are Jakhongir Fatikhovich Alidjanov (Uzbekistan), Ozoda Takhirovna Alidjanova (Uzbekistan), Adrian Martin Erich Pilatz (Germany), Kurt Guenther Naber (Germany), and Florian Martin Erich Wagenlehner (Germany). The e-USQOLAT is copyrighted by the Authorship Certificate of the International Online Copyright Office, European Depository, Berlin, Germany (Nr. EC-01-001179; 18 May 2017) 19. Translations of the ACSS in other languages are available on the website https://www.acss.world/downloads.html.
Competing interests
KGN, JA, AP, and FMW are authors and copyright holders of the ACSS questionnaire. KGN is a consultant of Adamed Pharma, Bionorica, BioMerieux, GlaxoSmithKline, Immunotek, Ingenion Medical, Janssen Pharmaceutica, OM Pharma, and MIP Pharma. JA is an employee of Bionorica SE. WLS is a consultant of Bionorica, Desitin, MIP Pharma. JK is a consultant of Bionorica and GSK. FMW is a consultant of Achaogen, Astellas, AstraZeneca, Bionorica, MSD, Eumedica, GSK, Janssen, Klosterfrau, MIP Pharma, Pfizer, OM Pharma, Qiagen, VenatoRx. RF declares no conflict of interest.
References
[1] Bonkat G, Bartoletti R, Bruyère F, Cai T, Geerlings SE, Köves B, Kranz J, Schubert S, Pilatz A, Veeratterapillay R, Wagenlehner F, Bausch K, Devlies W, Horváth J, Leitner L, Mantica G, Mezei T, Smith EJ. EAU Guidelines on Urological Infections. Ed. presented at the EAU Annual Congress in Milan, Italy. Arnhem, the Netherlands: EAU Guidelines Office; 2024. Available from: https://uroweb.org/guidelines/urological-infections[2] Wagenlehner FME, Bjerklund Johansen TE, Cai T, Koves B, Kranz J, Pilatz A, Tandogdu Z. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020;17(10):586-600. DOI: 10.1038/s41585-020-0362-4
[3] Butler CC, Hawking MK, Quigley A, McNulty CA. Incidence, severity, help seeking, and management of uncomplicated urinary tract infection: a population-based survey. Br J Gen Pract. 2015;65(639):e702-e707. DOI: 10.3399/bjgp15X686965
[4] Gupta K, Trautner BW. Diagnosis and management of recurrent urinary tract infections in non-pregnant women. BMJ. 2013;346:f3140. DOI: 10.1136/bmj.f3140
[5] Rich SN, Klann EM, Almond CR, Larkin EM, Nicolette G, Ball JD. Associations between antibiotic prescriptions and recurrent urinary tract infections in female college students. Epidemiol Infect. 2019 Jan;147:e119. DOI: 10.1017/S0950268818003369
[6] Hisano M, Bruschini H, Nicodemo AC, Srougi M. Uncomplicated Urinary Tract Infections in Women in a Sao Paulo Quaternary Care Hospital: Bacterial Spectrum and Susceptibility Patterns. Antibiotics (Basel). 2014 Mar 19;3(1):98-108. DOI: 10.3390/antibiotics3010098
[7] Nseir W, Farah R, Mahamid M, Sayed-Ahmad H, Mograbi J, Taha M, Artul S. Obesity and recurrent urinary tract infections in premenopausal women: a retrospective study. Int J Infect Dis. 2015 Dec;41:32-5. DOI: 10.1016/j.ijid.2015.10.014
[8] Little P, Merriman R, Turner S, Rumsby K, Warner G, Lowes JA, Smith H, Hawke C, Leydon G, Mullee M, Moore MV. Presentation, pattern, and natural course of severe symptoms, and role of antibiotics and antibiotic resistance among patients presenting with suspected uncomplicated urinary tract infection in primary care: observational study. BMJ. 2010 Feb 5;340:b5633. DOI: 10.1136/bmj.b5633
[9] Butler CC, Francis N, Thomas-Jones E, Llor C, Bongard E, Moore M, Little P, Bates J, Lau M, Pickles T, Gal M, Wootton M, Kirby N, Gillespie D, Rumbsy K, Brugman C, Hood K, Verheij T. Variations in presentation, management, and patient outcomes of urinary tract infection: a prospective four-country primary care observational cohort study. Br J Gen Pract. 2017 Dec;67(665):e830-e841. DOI: 10.3399/bjgp17X693641
[10] Kornfält Isberg H, Hedin K, Melander E, Mölstad S, Beckman A. Uncomplicated urinary tract infection in primary health care: presentation and clinical outcome. Infect Dis (Lond). 2021 Feb;53(2):94-101. DOI: 10.1080/23744235.2020.1834138
[11] Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011 Mar 1;52(5):e103-20. DOI: 10.1093/cid/ciq257
[12] Kang CI, Kim J, Park DW, Kim BN, Ha US, Lee SJ, Yeo JK, Min SK, Lee H, Wie SH. Clinical Practice Guidelines for the Antibiotic Treatment of Community-Acquired Urinary Tract Infections. Infect Chemother. 2018 Mar;50(1):67-100. DOI: 10.3947/ic.2018.50.1.67
[13] Japanese Association for Infectious Disease/Japanese Society of Chemotherapy; JAID/JSC Guide/Guidelines to Clinical Management of Infectious Disease Preparing Committee; Urinary tract infection/male genital infection working group; Yamamoto S, Ishikawa K, Hayami H, Nakamura T, Miyairi I, Hoshino T, Hasui M, Tanaka K, Kiyota H, Arakawa S. JAID/JSC Guidelines for Clinical Management of Infectious Disease 2015 - Urinary tract infection/male genital infection. J Infect Chemother. 2017 Nov;23(11):733-51. DOI: 10.1016/j.jiac.2017.02.002
[14] China Medical Women’s Association Special Committee on Kidney Disease and Blood Purification. Consensus of Chinese experts on the diagnosis and treatment of female urinary tract infection. Natl Med J Chin. 2017;97:2827-32.
[15] Haddad JM, Ubertazzi E, Cabrera OS, Medina M, Garcia J, Rodriguez-Colorado S, Toruno E, Matsuoka PK, Castillo-Pino E. Latin American consensus on uncomplicated recurrent urinary tract infection-2018. Int Urogynecol J. 2020 Jan;31(1):35-44. DOI: 10.1007/s00192-019-04079-5
[16] van Driel AA, Notermans DW, Meima A, Mulder M, Donker GA, Stobberingh EE, Verbon A. Antibiotic resistance of Escherichia coli isolated from uncomplicated UTI in general practice patients over a 10-year period. Eur J Clin Microbiol Infect Dis. 2019 Nov;38(11):2151-8. DOI: 10.1007/s10096-019-03655-3
[17] Kornfält Isberg H, Melander E, Hedin K, Mölstad S, Beckman A. Uncomplicated urinary tract infections in Swedish primary care; etiology, resistance and treatment. BMC Infect Dis. 2019 Feb 13;19(1):155. DOI: 10.1186/s12879-019-3785-x
[18] Ny S, Edquist P, Dumpis U, Gröndahl-Yli-Hannuksela K, Hermes J, Kling AM, Klingeberg A, Kozlov R, Källman O, Lis DO, Pomorska-Wesołowska M, Saule M, Wisell KT, Vuopio J, Palagin I; NoDARS UTIStudy Group. Antimicrobial resistance of Escherichia coli isolates from outpatient urinary tract infections in women in six European countries including Russia. J Glob Antimicrob Resist. 2019 Jun;17:25-34. DOI: 10.1016/j.jgar.2018.11.004
[19] Mulder M, Kiefte-de Jong JC, Goessens WH, de Visser H, Hofman A, Stricker BH, Verbon A. Risk factors for resistance to ciprofloxacin in community-acquired urinary tract infections due to Escherichia coli in an elderly population. J Antimicrob Chemother. 2017 Jan;72(1):281-9. DOI: 10.1093/jac/dkw399
[20] Stapleton AE, Wagenlehner FME, Mulgirigama A, Twynholm M. Escherichia coli Resistance to Fluoroquinolones in Community-Acquired Uncomplicated Urinary Tract Infection in Women: a Systematic Review. Antimicrob Agents Chemother. 2020 Sep 21;64(10):e00862-20. DOI: 10.1128/AAC.00862-20
[21] Stewardson AJ, Vervoort J, Adriaenssens N, Coenen S, Godycki-Cwirko M, Kowalczyk A, Huttner BD, Lammens C, Malhotra-Kumar S, Goossens H, Harbarth S; SATURN WP1 Study Group; SATURN WP3 Study Group. Effect of outpatient antibiotics for urinary tract infections on antimicrobial resistance among commensal Enterobacteriaceae: a multinational prospective cohort study. Clin Microbiol Infect. 2018 Sep;24(9):972-9. DOI: 10.1016/j.cmi.2017.12.026
[22] van Driel A. Antibiotic resistance of uropathogenic Escherichia coli and ESBL prevalence in general practice patients over 10 years. Br J Gen Pract. 2020 Jun;70(suppl 1):bjgp20X711533. DOI: 10.3399/bjgp20X711533
[23] Kaye KS, Gupta V, Mulgirigama A, Joshi AV, Scangarella-Oman NE, Yu K, Ye G, Mitrani-Gold FS. Antimicrobial Resistance Trends in Urine Escherichia coli Isolates From Adult and Adolescent Females in the United States From 2011 to 2019: Rising ESBL Strains and Impact on Patient Management. Clin Infect Dis. 2021 Dec 6;73(11):1992-9. DOI: 10.1093/cid/ciab560
[24] Yang Q, Zhang H, Wang Y, Xu Z, Zhang G, Chen X, Xu Y, Cao B, Kong H, Ni Y, Yu Y, Sun Z, Hu B, Huang W, Wang Y, Wu A, Feng X, Liao K, Luo Y, Hu Z, Chu Y, Lu J, Su J, Gui B, Duan Q, Zhang S, Shao H, Badal RE. Antimicrobial susceptibilities of aerobic and facultative gram-negative bacilli isolated from Chinese patients with urinary tract infections between 2010 and 2014. BMC Infect Dis. 2017 Mar 6;17(1):192. DOI: 10.1186/s12879-017-2296-x
[25] Zavala-Cerna MG, Segura-Cobos M, Gonzalez R, Zavala-Trujillo IG, Navarro-Perez SF, Rueda-Cruz JA, Satoscoy-Tovar FA. The Clinical Significance of High Antimicrobial Resistance in Community-Acquired Urinary Tract Infections. Can J Infect Dis Med Microbiol. 2020 Jun 4;2020:2967260. DOI: 10.1155/2020/2967260
[26] Muhammad A, Khan SN, Ali N, Rehman MU, Ali I. Prevalence and antibiotic susceptibility pattern of uropathogens in outpatients at a tertiary care hospital. New Microbes New Infect. 2020 Jun 13;36:100716. DOI: 10.1016/j.nmni.2020.100716
[27] US Food and Drug Administration (FDA). FDA updates warnings fluoroquinolone antibiotics. Silver Spring, MD: FDA; 2016 Jul 26 [cited 2023 Nov 3]. Available from: https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics
[28] US Food and Drug Administration (FDA). FDA updates warnings for fluoroquinolone antibiotics on risks of mental health and low blood sugar adverse reactions. Silver Spring, MD: FDA; 2018 Jul 10 [cited 2023 Nov 3]. Available from: https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse
[29] Wagenlehner F, Wullt B, Ballarini S, Zingg D, Naber KG. Social and economic burden of recurrent urinary tract infections and quality of life: a patient web-based study (GESPRIT). Expert Rev Pharmacoecon Outcomes Res. 2018 Feb;18(1):107-17. DOI: 10.1080/14737167.2017.1359543
[30] Price JR, Guran LA, Gregory WT, McDonagh MS. Nitrofurantoin vs other prophylactic agents in reducing recurrent urinary tract infections in adult women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016 Nov;215(5):548-60. DOI: 10.1016/j.ajog.2016.07.040
[31] Albert X, Huertas I, Pereiró II, Sanfélix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;2004(3):CD001209. DOI: 10.1002/14651858.CD001209.pub2
[32] Bjerrum L, Lindbæk M. Which treatment strategy for women with symptoms of urinary tract infection? BMJ. 2015 Dec 29;351:h6888. DOI: 10.1136/bmj.h6888
[33] Wagenlehner F, Nicolle L, Bartoletti R, Gales AC, Grigoryan L, Huang H, Hooton T, Lopardo G, Naber K, Poojary A, Stapleton A, Talan DA, Saucedo JT, Wilcox MH, Yamamoto S, Yang SS, Lee SJ. A global perspective on improving patient care in uncomplicated urinary tract infection: expert consensus and practical guidance. J Glob Antimicrob Resist. 2022 Mar;28:18-29. DOI: 10.1016/j.jgar.2021.11.008
[34] Kranz J, Schmidt S, Lebert C, Schneidewind L, Mandraka F, Kunze M, Helbig S, Vahlensieck W, Naber K, Schmiemann G, Wagenlehner FM. The 2017 Update of the German Clinical Guideline on Epidemiology, Diagnostics, Therapy, Prevention, and Management of Uncomplicated Urinary Tract Infections in Adult Patients: Part 1. Urol Int. 2018;100(3):263-70. DOI: 10.1159/000486138
[35] Johansen TE, Botto H, Cek M, Grabe M, Tenke P, Wagenlehner FM, Naber KG. Critical review of current definitions of urinary tract infections and proposal of an EAU/ESIU classification system. Int J Antimicrob Agents. 2011 Dec;38 Suppl:64-70. DOI: 10.1016/j.ijantimicag.2011.09.009
[36] US Department of Health and Human Services; Food and Drug Administration (FDA); Center for Drug Evaluation and Research (CDER). Uncomplicated Urinary Tract Infections: Developing Drugs for Treatment Guidance for Industry. Silver Spring, MD: FDA; 2019 Aug. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/uncomplicated-urinary-tract-infections-developing-drugs-treatment-guidance-industry
[37] European Medicines Agency (EMA); Committee for Medicinal Products for Human Use (CHMP). Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. Amsterdam: EMA; 2022. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-medicinal-products-indicated-treatment-bacterial-infections-revision-3_en.pdf
[38] Kass EH. Chemotherapeutic and antibiotic drugs in the management of infections of the urinary tract. Am J Med. 1955 May;18(5):764-81. DOI: 10.1016/0002-9343(55)90190-x
[39] Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982 Aug 19;307(8):463-8. DOI: 10.1056/NEJM198208193070802
[40] Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013 Nov 14;369(20):1883-91. DOI: 10.1056/NEJMoa1302186
[41] Heytens S, De Sutter A, Coorevits L, Cools P, Boelens J, Van Simaey L, Christiaens T, Vaneechoutte M, Claeys G. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin Microbiol Infect. 2017 Sep;23(9):647-52. DOI: 10.1016/j.cmi.2017.04.004
[42] Alidjanov JF, Abdufattaev UA, Makhmudov DK, Mirkhamidov DK, Khadzhikhanov FA, Azgamov AV, Pilatz A, Naber KG, Wagenlehner FM, Akilov FA. [Development and clinical testing of the Russian version of the Acute Cystitis Symptom Score - ACSS]. Urologiia. 2014 Nov-Dec;(6):14-22.
[43] Alidjanov JF, Naber KG, Pilatz A, Wagenlehner FM. Validation of the American English Acute Cystitis Symptom Score. Antibiotics (Basel). 2020 Dec 19;9(12):929. DOI: 10.3390/antibiotics9120929
[44] Alidjanov JF, Naber KG, Pilatz A, Radzhabov A, Zamuddinov M, Magyar A, Tenke P, Wagenlehner FM. Evaluation of the draft guidelines proposed by EMA and FDA for the clinical diagnosis of acute uncomplicated cystitis in women. World J Urol. 2020 Jan;38(1):63-72. DOI: 10.1007/s00345-019-02761-3
[45] Naber KG, Thyroff-Friesinger U. Fosfomycin trometamol versus ofloxacin/co-trimoxazole as single dose therapy of acute uncomplicated urinary tract infection in females: a multicentre study. Infection. 1990;18 Suppl 2:S70-6. DOI: 10.1007/BF01643431
[46] Spurbeck RR, Stapleton AE, Johnson JR, Walk ST, Hooton TM, Mobley HL. Fimbrial profiles predict virulence of uropathogenic Escherichia coli strains: contribution of ygi and yad fimbriae. Infect Immun. 2011 Dec;79(12):4753-63. DOI: 10.1128/IAI.05621-11
[47] Hunstad DA, Justice SS. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu Rev Microbiol. 2010;64:203-21. DOI: 10.1146/annurev.micro.112408.134258
[48] Fünfstück R, Wagenlehner FM, Olschläger T, Naber KG. Harnwegsinfektionen: Zystitis, Pyelonephritis, Urosepsis [Urinary tract infections: cystitis, pyelonephritis, urosepsis]. Dtsch Med Wochenschr. 2012 Feb;137(5):198-201. DOI: 10.1055/s-0031-1292886
[49] Fünfstück R, Franke S, Hellberg M, Ott U, Knöfel B, Straube E, Sommer M, Hacker J. Secretion of cytokines by uroepithelial cells stimulated by Escherichia coli and Citrobacter spp. Int J Antimicrob Agents. 2001 Apr;17(4):253-8. DOI: 10.1016/s0924-8579(01)00301-6
[50] Choe HS, Lee SJ, Yang SS, Hamasuna R, Yamamoto S, Cho YH, Matsumoto T; Committee for Development of the UAA-AAUS Guidelines for UTI and STI. Summary of the UAA-AAUS guidelines for urinary tract infections. Int J Urol. 2018 Mar;25(3):175-85. DOI: 10.1111/iju.13493
[51] Anger J, Lee U, Ackerman AL, Chou R, Chughtai B, Clemens JQ, Hickling D, Kapoor A, Kenton KS, Kaufman MR, Rondanina MA, Stapleton A, Stothers L, Chai TC. Recurrent Uncomplicated Urinary Tract Infections in Women: AUA/CUA/SUFU Guideline. J Urol. 2019 Aug;202(2):282-9. DOI: 10.1097/JU.0000000000000296
[52] Perepanova TS. Federal'nye klinicheskie rekomendatsii «Antimikrobnaya terapiya i profilaktika infektsii pochek, mochevyvodyashchikh putei i muzhskikh polovykh organov - 2015 g.» [The 2015 Federal Clinical Guidelines for Antimicrobial Therapy and Prevention of Infections of the Kidney, Urinary Tract, and Male Genitals]. Ter Arkh. 2016;88(4):100-4. DOI: 10.17116/terarkh2016884100-104
[53] Naber KG, Wagenlehner F, Kresken M, Cheng WY, Catillon M, Duh MS, Yu L, Khanal A, Mulgirigama A, Joshi AV, Ju S, Mitrani-Gold FS. Escherichia coli resistance, treatment patterns and clinical outcomes among females with uUTI in Germany: a retrospective physician-based chart review study. Sci Rep. 2023 Jul 26;13(1):12077. DOI: 10.1038/s41598-023-38919-8
[54] Kranz J, Schmidt S, Lebert C, Schneidewind L, Mandraka F, Kunze M, Helbig S, Vahlensieck W, Naber K, Schmiemann G, Wagenlehner FM. The 2017 Update of the German Clinical Guideline on Epidemiology, Diagnostics, Therapy, Prevention, and Management of Uncomplicated Urinary Tract Infections in Adult Patients. Part II: Therapy and Prevention. Urol Int. 2018;100(3):271-8. DOI: 10.1159/000487645
[55] Naber KG, Niggemann H, Stein G, Stein G. Review of the literature and individual patients' data meta-analysis on efficacy and tolerance of nitroxoline in the treatment of uncomplicated urinary tract infections. BMC Infect Dis. 2014 Nov 27;14:628. DOI: 10.1186/s12879-014-0628-7
[56] Wagenlehner F, Kresken M, Wohlfarth E, Bahrs C, Grabein B, Strohmaier WL, Naber KG. Therapie der Zystitis mit Nitroxolin – NitroxWin: Prospektive, multizentrische, nicht-interventionelle Studie und mikrobiologische Untersuchungen zur Resistenzsituation [Therapy of cystitis with nitroxoline – NitroxWin: Prospective, multicenter, non-interventional study and microbiological resistance surveillance]. Urologie. 2023 Nov;62(11):1186-92. DOI: 10.1007/s00120-023-02167-5
[57] Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012 Mar 15;366(11):1028-37. DOI: 10.1056/NEJMcp1104429
[58] Gágyor I, Bleidorn J, Kochen MM, Schmiemann G, Wegscheider K, Hummers-Pradier E. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ. 2015 Dec 23;351:h6544. DOI: 10.1136/bmj.h6544
[59] Kronenberg A, Bütikofer L, Odutayo A, Mühlemann K, da Costa BR, Battaglia M, Meli DN, Frey P, Limacher A, Reichenbach S, Jüni P. Symptomatic treatment of uncomplicated lower urinary tract infections in the ambulatory setting: randomised, double blind trial. BMJ. 2017 Nov 7;359:j4784. DOI: 10.1136/bmj.j4784
[60] Vik I, Bollestad M, Grude N, Bærheim A, Damsgaard E, Neumark T, Bjerrum L, Cordoba G, Olsen IC, Lindbæk M. Ibuprofen versus pivmecillinam for uncomplicated urinary tract infection in women-A double-blind, randomized non-inferiority trial. PLoS Med. 2018 May 15;15(5):e1002569. DOI: 10.1371/journal.pmed.1002569
[61] Bleidorn J, Gagyor I, Schmiemann G. Harnwegsinfektionen - sind immer Antibiotika notwendig? Nieren- und Hochdruckkrankheiten. 2021;50(4):162-5. DOI: 10.5414/NHX02164
[62] Bleidorn J, Hummers-Pradier E, Schmiemann G, Wiese B, Gágyor I. Recurrent urinary tract infections and complications after symptomatic versus antibiotic treatment: follow-up of a randomised controlled trial. Ger Med Sci. 2016 Feb 10;14:Doc01. DOI: 10.3205/000228
[63] Jansåker F, Li X, Vik I, Frimodt-Møller N, Knudsen JD, Sundquist K. The Risk of Pyelonephritis Following Uncomplicated Cystitis: A Nationwide Primary Healthcare Study. Antibiotics (Basel). 2022 Nov 24;11(12):1695. DOI: 10.3390/antibiotics11121695
[64] Wagenlehner FM, Abramov-Sommariva D, Höller M, Steindl H, Naber KG. Non-Antibiotic Herbal Therapy (BNO 1045) versus Antibiotic Therapy (Fosfomycin Trometamol) for the Treatment of Acute Lower Uncomplicated Urinary Tract Infections in Women: A Double-Blind, Parallel-Group, Randomized, Multicentre, Non-Inferiority Phase III Trial. Urol Int. 2018;101(3):327-36. DOI: 10.1159/000493368
[65] Butler DSC, Wagenlehner F, Höller M, Abramov-Sommariva D, Steindl H, Naber KG. Phytotherapy (BNO 1045) of Acute Lower Uncomplicated Urinary Tract Infection in Women Normalizes Local Host Responses. Urol Int. 2023;107(8):778-84. DOI: 10.1159/000531206
[66] Alidjanov JF, Naber KG, Pilatz A, Radzhabov A, Zamuddinov M, Magyar A, Tenke P, Wagenlehner FM. Additional assessment of Acute Cystitis Symptom Score questionnaire for patient-reported outcome measure in female patients with acute uncomplicated cystitis: part II. World J Urol. 2020 Aug;38(8):1977-88. DOI: 10.1007/s00345-019-02948-8
[67] Piontek K, Donhauser T, Kann G, Fechtner M, Apfelbacher C, Gabes M. Patient-reported outcome measures for uncomplicated urinary tract infections in women: a systematic review. Qual Life Res. 2023 Aug;32(8):2137-53. DOI: 10.1007/s11136-023-03358-5
[68] Cai T. Recurrent uncomplicated urinary tract infections: definitions and risk factors. GMS Infect Dis. 2021 May 27;9:Doc03. DOI: 10.3205/id000072
[69] Scholes D, Hooton TM, Roberts PL, Stapleton AE, Gupta K, Stamm WE. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000 Oct;182(4):1177-82. DOI: 10.1086/315827
[70] Lüthje P, Brauner H, Ramos NL, Ovregaard A, Gläser R, Hirschberg AL, Aspenström P, Brauner A. Estrogen supports urothelial defense mechanisms. Sci Transl Med. 2013 Jun 19;5(190):190ra80. DOI: 10.1126/scitranslmed.3005574
[71] Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993 Sep 9;329(11):753-6. DOI: 10.1056/NEJM199309093291102
[72] Cai T, Mazzoli S, Migno S, Malossini G, Lanzafame P, Mereu L, Tateo S, Wagenlehner FM, Pickard RS, Bartoletti R. Development and validation of a nomogram predicting recurrence risk in women with symptomatic urinary tract infection. Int J Urol. 2014 Sep;21(9):929-34. DOI: 10.1111/iju.12453
[73] Bermingham SL, Ashe JF. Systematic review of the impact of urinary tract infections on health-related quality of life. BJU Int. 2012 Dec;110(11 Pt C):E830-6. DOI: 10.1111/j.1464-410X.2012.11337.x
[74] Naber KG, Tirán-Saucedo J, Wagenlehner FME; RECAP group. Psychosocial burden of recurrent uncomplicated urinary tract infections. GMS Infect Dis. 2022 Mar 24;10:Doc01. DOI: 10.3205/id000078
[75] Flower A, Bishop FL, Lewith G. How women manage recurrent urinary tract infections: an analysis of postings on a popular web forum. BMC Fam Pract. 2014 Sep 26;15:162. DOI: 10.1186/1471-2296-15-162
[76] Cai T, Mazzoli S, Mondaini N, Meacci F, Nesi G, D'Elia C, Malossini G, Boddi V, Bartoletti R. The role of asymptomatic bacteriuria in young women with recurrent urinary tract infections: to treat or not to treat? Clin Infect Dis. 2012 Sep;55(6):771-7. DOI: 10.1093/cid/cis534
[77] Sundén F, Håkansson L, Ljunggren E, Wullt B. Escherichia coli 83972 bacteriuria protects against recurrent lower urinary tract infections in patients with incomplete bladder emptying. J Urol. 2010 Jul;184(1):179-85. DOI: 10.1016/j.juro.2010.03.024
[78] Naboka YL, Mavzyiutov AR, Kogan MI, Gudima IA, Ivanov SN, Naber KG. Does Escherichia coli have pathogenic potential at a low level of bacteriuria in recurrent, uncomplicated urinary tract infection? Int J Antimicrob Agents. 2020 Jul;56(1):105983. DOI: 10.1016/j.ijantimicag.2020.105983
[79] Naboka YL, Mavzyutov AR, Kogan MI, Gudima IA, Dzhalagoniya KT, Ivanov SN, Naber KG. The gene profile of Enterobacteriaceae virulence factors in relation to bacteriuria levels between the acute episodes of recurrent uncomplicated lower urinary tract infection. Expert Rev Anti Infect Ther. 2021 Aug;19(8):1061-6. DOI: 10.1080/14787210.2021.1866986
[80] Hooton TM, Vecchio M, Iroz A, Tack I, Dornic Q, Seksek I, Lotan Y. Effect of Increased Daily Water Intake in Premenopausal Women With Recurrent Urinary Tract Infections: A Randomized Clinical Trial. JAMA Intern Med. 2018 Nov 1;178(11):1509-15. DOI: 10.1001/jamainternmed.2018.4204
[81] Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012 Oct 17;10(10):CD001321. DOI: 10.1002/14651858.CD001321.pub5
[82] Beerepoot MA, Geerlings SE, van Haarst EP, van Charante NM, ter Riet G. Nonantibiotic prophylaxis for recurrent urinary tract infections: a systematic review and meta-analysis of randomized controlled trials. J Urol. 2013 Dec;190(6):1981-9. DOI: 10.1016/j.juro.2013.04.142
[83] Beerepoot M, Geerlings S. Non-Antibiotic Prophylaxis for Urinary Tract Infections. Pathogens. 2016 Apr 16;5(2):36. DOI: 10.3390/pathogens5020036
[84] Fu Z, Liska D, Talan D, Chung M. Cranberry Reduces the Risk of Urinary Tract Infection Recurrence in Otherwise Healthy Women: A Systematic Review and Meta-Analysis. J Nutr. 2017 Dec;147(12):2282-8. DOI: 10.3945/jn.117.254961
[85] Luís Â, Domingues F, Pereira L. Can Cranberries Contribute to Reduce the Incidence of Urinary Tract Infections? A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Clinical Trials. J Urol. 2017 Sep;198(3):614-21. DOI: 10.1016/j.juro.2017.03.078
[86] Xia JY, Yang C, Xu DF, Xia H, Yang LG, Sun GJ. Consumption of cranberry as adjuvant therapy for urinary tract infections in susceptible populations: A systematic review and meta-analysis with trial sequential analysis. PLoS One. 2021 Sep 2;16(9):e0256992. DOI: 10.1371/journal.pone.0256992
[87] Kranz J, Lackner J, Künzel U, Wagenlehner F, Schmidt S. Phytotherapy in Adults With Recurrent Uncomplicated Cystitis. Dtsch Arztebl Int. 2022 May 20;119(20):353-60. DOI: 10.3238/arztebl.m2022.0104
[88] Williams G, Hahn D, Stephens JH, Craig JC, Hodson EM. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2023 Apr 17;4(4):CD001321. DOI: 10.1002/14651858.CD001321.pub6
[89] Gupta K, Chou MY, Howell A, Wobbe C, Grady R, Stapleton AE. Cranberry products inhibit adherence of p-fimbriated Escherichia coli to primary cultured bladder and vaginal epithelial cells. J Urol. 2007 Jun;177(6):2357-60. DOI: 10.1016/j.juro.2007.01.114
[90] Howell AB, Botto H, Combescure C, Blanc-Potard AB, Gausa L, Matsumoto T, Tenke P, Sotto A, Lavigne JP. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect Dis. 2010 Apr 14;10:94. DOI: 10.1186/1471-2334-10-94
[91] Babar A, Moore L, Leblanc V, Dudonné S, Desjardins Y, Lemieux S, Bochard V, Guyonnet D, Dodin S. High dose versus low dose standardized cranberry proanthocyanidin extract for the prevention of recurrent urinary tract infection in healthy women: a double-blind randomized controlled trial. BMC Urol. 2021 Mar 23;21(1):44. DOI: 10.1186/s12894-021-00811-w
[92] Albrecht U, Goos KH, Schneider B. A randomised, double-blind, placebo-controlled trial of a herbal medicinal product containing Tropaeoli majoris herba (Nasturtium) and Armoraciae rusticanae radix (Horseradish) for the prophylactic treatment of patients with chronically recurrent lower urinary tract infections. Curr Med Res Opin. 2007 Oct;23(10):2415-22. DOI: 10.1185/030079907X233089
[93] Wagenlehner F, Lorenz H, Ewald O, Gerke P. Why d-Mannose May Be as Efficient as Antibiotics in the Treatment of Acute Uncomplicated Lower Urinary Tract Infections-Preliminary Considerations and Conclusions from a Non-Interventional Study. Antibiotics (Basel). 2022 Feb 25;11(3):314. DOI: 10.3390/antibiotics11030314
[94] Kranjčec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014 Feb;32(1):79-84. DOI: 10.1007/s00345-013-1091-6
[95] Lenger SM, Bradley MS, Thomas DA, Bertolet MH, Lowder JL, Sutcliffe S. D-mannose vs other agents for recurrent urinary tract infection prevention in adult women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020 Aug;223(2):265.e1-265.e13. DOI: 10.1016/j.ajog.2020.05.048
[96] Kyriakides R, Jones P, Somani BK. Role of D-Mannose in the Prevention of Recurrent Urinary Tract Infections: Evidence from a Systematic Review of the Literature. Eur Urol Focus. 2021 Sep;7(5):1166-9. DOI: 10.1016/j.euf.2020.09.004
[97] Cooper TE, Teng C, Howell M, Teixeira-Pinto A, Jaure A, Wong G. D-mannose for preventing and treating urinary tract infections. Cochrane Database Syst Rev. 2022 Aug 30;8(8):CD013608. DOI: 10.1002/14651858.CD013608.pub2
[98] Bessler WG, vor dem Esche U, Zgaga-Griesz A, Ataullakhanov R. Immunostimulatory properties of the bacterial extract OM-89 in vitro and in vivo. Arzneimittelforschung. 2010;60(6):324-9. DOI: 10.1055/s-0031-1296295
[99] Naber KG, Cho YH, Matsumoto T, Schaeffer AJ. Immunoactive prophylaxis of recurrent urinary tract infections: a meta-analysis. Int J Antimicrob Agents. 2009 Feb;33(2):111-9. DOI: 10.1016/j.ijantimicag.2008.08.011
[100] Aziminia N, Hadjipavlou M, Philippou Y, Pandian SS, Malde S, Hammadeh MY. Vaccines for the prevention of recurrent urinary tract infections: a systematic review. BJU Int. 2019 May;123(5):753-68. DOI: 10.1111/bju.14606
[101] Nestler S, Grüne B, Schilchegger L, Suna A, Perez A, Neisius A. Efficacy of vaccination with StroVac for recurrent urinary tract infections in women: a comparative single-centre study. Int Urol Nephrol. 2021 Nov;53(11):2267-72. DOI: 10.1007/s11255-021-02987-4
[102] Nestler S, Peschel C, Horstmann AH, Vahlensieck W, Fabry W, Neisius A. Prospective multicentre randomized double-blind placebo-controlled parallel group study on the efficacy and tolerability of StroVac® in patients with recurrent symptomatic uncomplicated bacterial urinary tract infections. Int Urol Nephrol. 2023 Jan;55(1):9-16. DOI: 10.1007/s11255-022-03379-y
[103] Nickel JC, Saz-Leal P, Doiron RC. Could sublingual vaccination be a viable option for the prevention of recurrent urinary tract infection in Canada? A systematic review of the current literature and plans for the future. Can Urol Assoc J. 2020 Aug;14(8):281-7. DOI: 10.5489/cuaj.6690
[104] Lorenzo-Gómez MF, Foley S, Nickel JC, García-Cenador MB, Padilla-Fernández BY, González-Casado I, Martínez-Huélamo M, Yang B, Blick C, Ferreira F, Caballero R, Saz-Leal P, Casanovas M. Sublingual MV140 for Prevention of Recurrent Urinary Tract Infections. NEJM Evid. 2022 Apr;1(4):EVIDoa2100018. DOI: 10.1056/EVIDoa2100018
[105] Falagas ME, Betsi GI, Tokas T, Athanasiou S. Probiotics for prevention of recurrent urinary tract infections in women: a review of the evidence from microbiological and clinical studies. Drugs. 2006;66(9):1253-61. DOI: 10.2165/00003495-200666090-00007
[106] Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, Yarova-Yarovaya Y, Fiedler T, Cox M, Stamm WE. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011 May;52(10):1212-7. DOI: 10.1093/cid/cir183
[107] Baerheim A, Larsen E, Digranes A. Vaginal application of lactobacilli in the prophylaxis of recurrent lower urinary tract infection in women. Scand J Prim Health Care. 1994 Dec;12(4):239-43. DOI: 10.3109/02813439409029247
[108] Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Koskela M, Uhari M. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ. 2001 Jun 30;322(7302):1571. DOI: 10.1136/bmj.322.7302.1571
[109] Beerepoot MA, ter Riet G, Nys S, van der Wal WM, de Borgie CA, de Reijke TM, Prins JM, Koeijers J, Verbon A, Stobberingh E, Geerlings SE. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012 May 14;172(9):704-12. DOI: 10.1001/archinternmed.2012.777
[110] Cai T, Cocci A, Tiscione D, Puglisi M, Di Maida F, Malossini G, Verze P, Palmieri A, Mirone V, Bjerklund Johansen TE. L-Methionine associated with Hibiscus sabdariffa and Boswellia serrata extracts are not inferior to antibiotic treatment for symptoms relief in patients affected by recurrent uncomplicated urinary tract infections: Focus on antibiotic-sparing approach. Arch Ital Urol Androl. 2018 Jun 30;90(2):97-100. DOI: 10.4081/aiua.2018.2.97
[111] Cai T, Anceschi U, Tamanini I, Migno S, Rizzo M, Liguori G, Garcia-Larrosa A, Palmieri A, Verze P, Mirone V, Bjerklund Johansen TE. Xyloglucan, Hibiscus and Propolis in the Management of Uncomplicated Lower Urinary Tract Infections: A Systematic Review and Meta-Analysis. Antibiotics (Basel). 2021 Dec 23;11(1):14. DOI: 10.3390/antibiotics11010014
[112] Dimitrakov J, Kroenke K, Steers WD, Berde C, Zurakowski D, Freeman MR, Jackson JL. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med. 2007 Oct 8;167(18):1922-9. DOI: 10.1001/archinte.167.18.1922
[113] Parsons CL. The therapeutic role of sulfated polysaccharides in the urinary bladder. Urol Clin North Am. 1994 Feb;21(1):93-100.
[114] Tseng CS, Chang SJ, Meng E, Chang HC, Lee YJ. The efficacy of pentosan polysulfate monotherapy for preventing recurrent urinary tract infections in women: A multicenter open-label randomized controlled trial. J Formos Med Assoc. 2020 Aug;119(8):1314-9. DOI: 10.1016/j.jfma.2019.11.007
[115] Harding C, Mossop H, Homer T, Chadwick T, King W, Carnell S, Lecouturier J, Abouhajar A, Vale L, Watson G, Forbes R, Currer S, Pickard R, Eardley I, Pearce I, Thiruchelvam N, Guerrero K, Walton K, Hussain Z, Lazarowicz H, Ali A. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, non-inferiority trial. BMJ. 2022 Mar 9;376:e068229. DOI: 10.1136/bmj-2021-0068229
[116] Damiano R, Quarto G, Bava I, Ucciero G, De Domenico R, Palumbo MI, Autorino R. Prevention of recurrent urinary tract infections by intravesical administration of hyaluronic acid and chondroitin sulphate: a placebo-controlled randomised trial. Eur Urol. 2011 Apr;59(4):645-51. DOI: 10.1016/j.eururo.2010.12.039
[117] De Vita D, Giordano S. Effectiveness of intravesical hyaluronic acid/chondroitin sulfate in recurrent bacterial cystitis: a randomized study. Int Urogynecol J. 2012 Dec;23(12):1707-13. DOI: 10.1007/s00192-012-1794-z
[118] Goddard JC, Janssen DAW. Intravesical hyaluronic acid and chondroitin sulfate for recurrent urinary tract infections: systematic review and meta-analysis. Int Urogynecol J. 2018 Jul;29(7):933-42. DOI: 10.1007/s00192-017-3508-z
[119] Naber KG, Alidjanov JF, Fünfstück R, Strohmaier WL, Kranz J, Cai T, Pilatz A, Wagenlehner FM. Therapeutic strategies for uncomplicated cystitis in women. GMS Infect Dis. 2024;12:Doc01. DOI: 10.3205/id000086
Attachments
| Attachment 1 | lhuii000073_att1 |
| Type | application/pdf |
| MD5 | 64d08a3e993ad718e35018fe505d61d5 |
| SHA256 | 83cd6c38d8c70851439ea29043d2fc753895fe4f714226b37959a182d65a0495 |




