Surgical treatment of urogenital tuberculosis
André Figueiredo 2
1 Department of Urology, R G Stone and Super Specialty Hospital, Ludhiana, India
2 Surgery, Federal University of Juiz de Fora, Brazil, Juiz de Fora, Brazil
Abstract
Various operative procedures have been described for urogenital tuberculosis (UGTB). These include percutaneous drainage of hydronephrosis or abscesses, double-J stenting for hydronephrosis, definitive local extirpative treatment of the affected part of a kidney (cavernotomy/partial nephrectomy) and epididymis (epididymectomy). Many forms of reconstructive surgery of the upper urinary tract (uretero-calycostomy, ureteric reimplantation, ileal ureteric replacement) and the lower urinary tract have been described. Nephrectomy is performed in 27% of UGTB patients and the frequency is similar in developing and developed countries. Radical and reconstructive procedures should be done within the first two months of intensive chemotherapy. A contracted bladder may need augmentation cystoplasty or even cystectomy if the capacity is less than 100 ml.
Summary of recommendations
Kidney and ureteral TB
- Excision of the affected tissue, including total or partial nephrectomy of a non-functioning kidney, is indicated if pain is persistent, recurrent secondary bacterial infections occur, severe hypertension develops or in case of coexisting renal cell carcinoma (LoE 4).
- Radical and reconstructive procedures should be done within the first two months of intensive chemotherapy (LoE 3).
- Ureteral obstruction in UGTB should be treated with stents or nephrostomy tubes which decreases the likelihood of renal loss (LoE 2a).
- Ureteral reimplantation is indicated in case of stenosis but may not be done in reflux cases (GoR B).
Contracted bladder
- Bladder augmentation with an intestinal segment is indicated when the bladder capacity is less than 100 ml (GoR B).
- In very small bladders (capacity less than 20 ml) or contracted bladders with pain (suprapubic or perineal), an orthotopic neobladder with cystectomy may be performed (GoR C).
- Ileum, sigmoid and the ileocecal segment can be used. Detubularization and reconfiguration should be performed, but ileocecal segment can be used in the tubularized original form (GoR B).
- Most patients can void spontaneously after bladder augmentation or neobladder. In case of high post-void residual urine, a prostatic resection (in patients older than 45 years) or urethral surgery (in urethral stenosis) should be performed (GoR B).
Tuberculous epididymo-orchitis
Surgical resection of tuberculous epididymo-orchitis may be performed through inguinal incision:
- If there is suspicion of tumor due to a solid scrotal mass, in this case through inguinal incision (GoR A).
- If control of local disease is needed in case of an abscess, scrotal fistula or persistent local pain (GoR B).
- If diagnostic improvement is needed when pathologic or microbiologic examinations are negative in patients with clinical suspicion of tuberculosis (GoR B).
1 Introduction
UGTB is third commonest form of extrathoracic TB, occuring due to metastatic spread of TB micro-organisms through the bloodstream during the initial infection. It affects males and females equally and is most commonly seen in the fourth decade of life. Insidiousness of onset and difficulty in diagnosis may lead to a delay in treatment. This may result in serious complications such as the destruction of kidney or severe affection of the urinary bladder. Surgery continues to play an important role in the management of UGTB, despite the availability of effective antituberculous drugs. Surgery may comprise excision of affected tissue such as nephrectomy or epididymectomy or as a reconstructive therapy such as enterocystoplasty and ureteric reimplantation in cases with ureteric or urethral strictures and contracted bladder.
2 Methods
A systematic literature search was performed for the last 10 years (2005–2015) in MEDLINE with the following key words: “Urogenital tuberculosis” without any limitations and “Urogenital Tuberculosis Surgery” in the English language. A total of 691 publications were identified, and screened by title and abstract. After exclusion of duplicates, a total of 38 were included in the analysis.
3 Results
Operative procedures for renal and ureteral TB can be categorized into five groups [1]:
- percutaneous drainage for hydronephrosis or abscesses or double-J stenting for hydronephrosis;
- endourological management of ureteral strictures;
- definitive local treatment of the affected part of the kidney (cavernotomy/partial nephrectomy);
- laparoscopic or open nephrectomy of a non-functioning kidney;
- reconstructive surgery of the upper urinary tract (uretero-calycostomy, ureteric reimplantation, ileal ureteric replacement).
3.1 Percutaneous drainage or double-J stenting
Ureteral obstruction in UGTB is treated with stents or nephrostomy tubes as clinically indicated.
Tuberculosis may cause obstructive kidney injury, which can be reversible after specific treatment in due time. There may be hydronephrosis secondary to stricture involving the pelvi-ureteric junction or the ureter. Such strictures will heal by fibrosis formation. DJ stenting may prevent further worsening by acting as a splint across the site of stricture. The strictures should be monitored with CT or IVU. If there is deterioration or no improvement after a six-week period, then surgical intervention is indicated and, in case of strictures of the lower ureter, reimplantation or endourological procedures including balloon dilatation may be necessary [1].
The use of corticosteroids in addition to stenting for ureteral obstruction is discussed in the literature, but its efficacy in this setting remains unclear. According to the latest Center for Disease Control guidelines, corticosteroids are not recommended for the treatment of UGTB [2].
Placement of a dJ stent is not always successful. In one series retrograde stent placement was successful in only 41% cases [3]. When a stent placement fails, a nephrostomy must be considered. Sometimes the disease may result in multiple infundibular stenoses or a scarred and cicatrized renal pelvis. In such instances, a percutaneous drainage of the cavity under ultrasound or CT guidance is recommended, along with continuation of the antituberculous therapy. This is a satisfactory method of initial treatment and postpones the need for surgery. It also allows the contents to be cultured for viable organisms. If the dJ stent placement fails, if the obstruction is getting worse, or in patients with persistent need of a nephrostomy, some type of open/laparoscopic reconstructive procedure is needed [3]. Both radical and reconstructive procedures should be done within the first two months of intensive chemotherapy [4].
3.2 Endourological management of ureteral strictures
3.2.1 Balloon dilation (retrograde or antegrade)
This treatment can be done only if it is possible to pass a guide wire through the stricture with either transurethral techniques or antegrade if a nephrostomy tube is in situ. These techniques are rarely definitive probably due to endarteritis induced ischemia and patients may need repeated dilatation. Balloon dilation should be reserved for patients with strictures less than 2 cm. Various authors have reported success rates varying from 50–60% [5], [6], [7], [8].
3.2.2 Endoureterotomy and cautery wire balloon incision
These procedures are performed under fluoroscopic control and should be avoided when the stricture is near great vessels, such as at the iliac level of the ureter. The ureterotomy incision can be made using a cold knife, a cutting electrode or laser. The incision should be made from the ureteral lumen out to periureteral fat in the full thickness of the ureteral wall. The incision should be made anteromedially in lower ureteric strictures and posterolaterally in upper ureteric strictures to avoid great vessels. Good prognostic features are length of stricture <1.5 cm, non-ischemic nature of stricture, and adequate renal function [4].
3.3 Definitive local treatment of the affected part of the kidney
3.3.1 Cavernotomy
There may be extensive cavitations at the poles of kidney, and the involved calyces do not excrete contrast medium. Ultrasound or CT abdomen usually helps diagnose the condition. Flank abscesses may form, which can grow to large sizes, especially if secondary infection sets in. These abscesses may rupture and spread into the perirenal space and beyond. Tubercular renal abscesses are seen as hypodense areas of 10–40 HU with mild peripheral enhancement. Cavitation within the renal parenchyma may be seen as irregular pools of contrast material if a calyceal communication exists. Focal, segmental, and polar involvement can be demonstrated [9]. Earlier, cavernotomy was frequently done because of ischemia of the involved calyx and inability of anti-tuberculous drugs to reach the ischemic site. With the availability of better imaging modalities, this procedure hardly has any place in the modern management of UGTB [10].
3.3.2 Partial and total nephrectomy
Excision of the affected tissue, including total or partial nephrectomy of a non-functioning kidney, is indicated if pain is ongoing, recurrent secondary bacterial infections occur or severe hypertension develops. The indication for partial nephrectomy is an area of calcification that is slowly increasing in size and threatening to gradually destroy the entire kidney [11].
Before the drug treatment era, nephrectomy was advocated for all calcified tuberculous kidneys, as it was thought that there was a high risk of reactivation. Currently, only a proportion of patients require a nephrectomy to prevent future complications. Some researchers advocate that surgical intervention should only be performed four weeks after the initiation of TB treatment, but there is no evidence to support this idea [10]. Nephrectomy is performed in non-functioning kidneys, renal tuberculosis with involvement of the entire renal parenchyma due to hypertension and ureteropelvic obstruction, as well as cases accompanied by renal cell carcinoma [12]. Nephrectomy improves hypertension in 65% of patients [13]. The indication for nephrectomy in a non-functional asymptomatic tuberculous kidney is still debatable. Since there is always a possibility of reactivation later in life, it is better to remove calcified and non-functional kidney [1].
Laparoscopic surgery for UGTB is usually more difficult than open surgery because of inflammation and fibrosis associated with the disease. There is more blood loss, a higher rate of conversion to open surgery, and a longer operative duration, putting stress on the surgical team and the anaesthetist because of difficult decision making and keeping a balance between the patient’s safety and benefit. Although laparoscopy is difficult, it is still an option for ablative procedure in UGTB. It offers the benefits of minimally invasive surgery and many authors have recommended retroperitoneoscopic or laparoscopic nephrectomy in UGTB [14], [15], [16]. A large series of 51 patients were managed with retroperitoneoscopic nephrectomy with only one conversion to open surgery. They advised to remove the entire ureter by open approach by Gibson incision because of ureteral stump syndrome [17].
If extensive fibrosis is present the traditional approach is through an oblique retroperitoneal incision which can be extended dorsally or ventrally as needed. The principles for doing nephrectomy for kidney tuberculosis are:
- Approach the kidney from behind because of colonic adhesions anteriorly.
- No need to remove the ureter completely, remove as much as possible.
- Try to ligate the artery and the vein separately in order to prevent arterio-venous fistula formation which might occur if both are tied together [18].
Overall, nephrectomy is performed in 27% of GUTB patients and the frequency is similar in developing and developed countries [19]. The clinical manifestations of renal TB are commonly unilateral and involve approximately 3% of all patients with TB [20].
3.3.3 Open surgical options in ureteropelvic and ureteral surgery
Reconstructive surgery for UGTB is required for cases with grossly distorted anatomy and impaired function that are unlikely to regress with chemotherapy alone [5]. Reconstructive surgery has a role in the management of UGTB, despite the presence of effective anti-tuberculous treatment [21].
Long, complex strictures require open surgical repair. Because of fibrosis/loss of elasticity in the dilated segment of the ureter in TB, it may not be possible to mobilize the ureter. Repair of UPJ scarring due to TB is more difficult than repair of a congenital stenosis. Dismembered pyeloplasty is feasible in extra renal pelvis with short segment of scarring. Non-dismembered (flap) pyeloplasty is preferred for longer strictures but may not be possible because of scarring [22]. Many of these problems requiring surgical intervention can now be managed by laparoscopic techniques, providing the benefits of minimally invasive surgery to the patient [14].
3.3.4 Ureteroureterostomy
A short defect involving the upper or the mid ureter can be treated by ureteroureterostomy. The anastomosis should be tension-free, hence enough ureteral mobility is an essential prerequisite [3]. Alternatively, lysis of adhesions and intubation (Davis intubated urethrotomy) can be done. This procedure was popular for long upper and mid-ureteric strictures wherein the incised ureter was left to heal by regeneration over a stent [21].
3.3.5 Ureteropyelostomy/ureterocalicostomy
Surgery for stricture at the pelviureteric junction or upper ureter is uncommon as the renal destruction is so severe in such cases that usually reconstruction is not possible. When anatomic reconstruction is not possible, ureterocalicostomy is an option. Ureterocalicostomy is preferred as there is often an associated calyceal dilatation. The renal capsule should be preserved to cover the lower pole of the kidney and if capsule is not available, omentum can be used to avoid stenosis at the site of the uretero-calyceal anastomosis [22].
3.3.6 Boaris flap and psoas hitch
Strictures at the lower end of the ureter can be managed conservatively with chemotherapy, corticosteroid treatment and dilatation or, if there is obstruction or impaired renal function, surgical intervention [11]. The strictured segment should be excised back to healthy mucosa. A Psoas hitch can bridge up to 5 cm and a Boaris flap up to 15 cm.
Gupta et al. have described laparoscopic ureteric reimplantation with the Psoas hitch procedure. In their experience, laparoscopic mobilization of the ureter was found to be easier, as is the dissection around the perivesical area, in which the adhesions were less often encountered and were less dense [14]. Long ureteric strictures involving almost the whole length of ureter and upper ureteric strictures require ileal replacement of ureter.
In a review of 39 series of UGTB, it was observed that close to half of the patients (54.9%) undergo surgery, a figure that ranges from 8–95%. In series where surgery was less frequent the patients were diagnosed while still asymptomatic or with fewer renal lesions. On the other hand, when the diagnosis is delayed, a silent progression of the disease may lead to organ destruction, with a subsequent greater frequency of surgical interventions [1].
Surgery may be ablative, with removal of the tuberculosis-destroyed kidney or epididymis, or reconstructive, with unblocking of the collecting system or augmentation of the contracted bladder [20]. A study from Russia which compared 209 UGTB patients operated between 1985 and 1987 to 188 patients operated between 2005 and 2007, showed a decrease of organ-removing surgery (61.1% to 27.3%) and an increase in reconstructive surgery (9.7% to 23%) [24].
3.4 Contracted bladder
Bladder tuberculosis is secondary to kidney tuberculosis and is caused by descendent urinary dissemination. Although urogenital tuberculosis has no specific initial symptoms or radiological findings, bladder tuberculosis has a characteristic clinical and radiological finding: the contracted bladder. In a review of published series of urogenital tuberculosis, contracted bladder was found in 8.9% of urogenital tuberculosis cases, but with a difference between developed (4.0%) and developing countries (13.6%) [23]. Radiologically, the contracted bladder presents with diffuse thickening of the bladder wall, without trabeculations or diverticulas. The vesicoureteral junction is located in the lateral upper part of the bladder, and it looks like the whole bladder has contracted except for the trigone [25] (Figure 1). Clinically, the patient presents with high urinary frequency, with a voiding interval of less than 20 minutes and a bladder capacity of less than 100 ml. Sometimes incontinence develops. The presence of a contracted bladder represents an advanced stage of urogenital tuberculosis infection. There is a hypothesis based on radiographic findings that tuberculosis involvement of the urinary tract may be sequential. After unilateral renal and ureteral involvement with thickening and stenosis of the collecting system with hydronephrosis and renal parenchyma atrophy and eventually loss of function, bladder damage may follow, with diffuse thickening of the bladder wall and development of secondary vesicoureteral reflux, usually unilateral to the still unaffected kidney. High-grade reflux may lead to ureterohydronephrosis, reflux nephropathy and risk of end-stage renal failure [25]. The mechanism that vesicoureteral reflux cause hydronephrosis and kidney damage leading to terminal renal failure was described already in 1969 [26], [27]. Therefore, patients with contracted bladder might present with tuberculous kidney disease and even terminal renal failure. The typical finding is a contracted bladder with unilateral ureteral obstruction (prone to re-implantation) or a non-function (prone to nephrectomy) kidney. The other kidney is usually normal or with hydronephrosis secondary to vesicoureteral reflux [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36] (Figure 1).
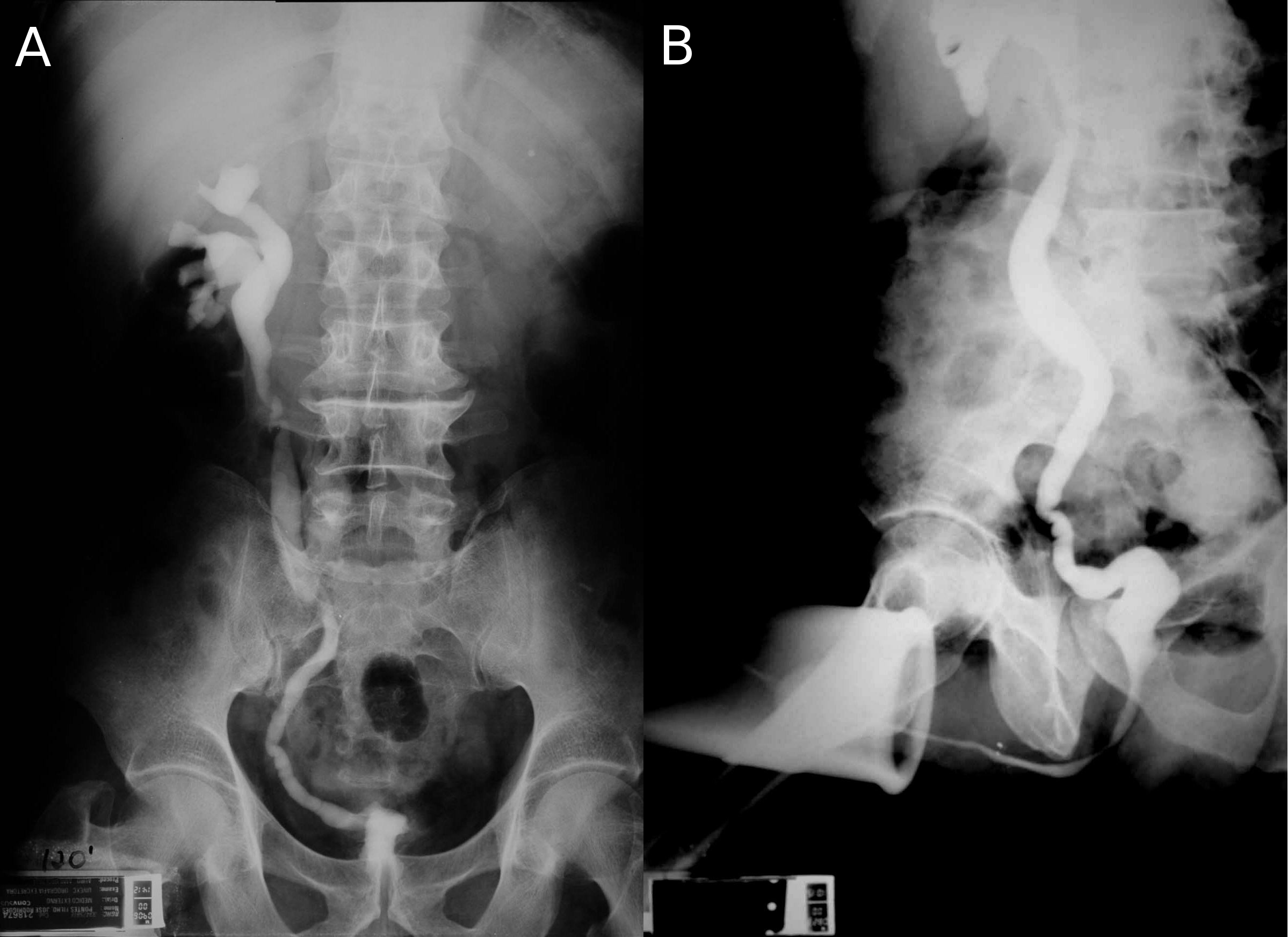
We found eleven case series of surgery for tuberculous contracted bladder [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]. The first series was published in 1969 and the most recent in 2014. Among 316 patients, 64% were men and the median age was between 30 and 40 years. There was an obvious lack of uniform description of patient data, however 60% of the patients had unilateral non-function kidney due to tuberculous destruction. In most series, the great majority of patients had unilateral non-function kidney (50–100%) but in two series there were only 17.9% and 30.7%, both from India [33], [35]. Bladder augmentation was performed in 90% of the cases and orthotopic neobladder was performed in 10%. In only three exceptional cases, a cutaneous urethrostomy was done [33]. Among bladder augmentations, ileum was used in 35.4% of cases with detubularization in all but a few cases in the two oldest series in 1969 and 1970. Sigmoid was used in 38.9% with detubularization in almost all cases and the ileocecal segment was used in 25.8%, however in the tubularized original form was used in almost all cases. The frequent use of sigmoid and ileocecal segment is explained by the need of ureteral reimplantation in cases of ureteral stenosis or high grade reflux. In the neobladder surgery, the same intestinal segments were used but the Studer procedure was used in 73.3%. The success criteria were not uniform among the series, but it was usually defined by improvement of micturition frequency and preservation of the upper urinary tract. The former was achieved in 80 to 100% of the cases. However, there were cases with progression to terminal renal failure in the same series [26], [31], [33], [34], [36] despite the absence of post-voiding residual and no stenosis or reflux of the uretero-vesical anastomosis. Different from bladder augmentation due to neurogenic bladder, most patients can void spontaneously with no need of self-catheterization. In 85.8% of cases, patients can void after surgery, and this improved to 94.2% after another surgery for obstruction such as transurethral prostate resection. In two series [29], [34] urodynamic evaluation was performed after surgery. Pressure flow studies showed that all patients voided by means of voluntary increase of abdominal pressure (Valsalva’s maneuver). However, in some cases Valsalva’s maneuver occurred during the involuntary contraction and the patient used this contraction to void. In cystometry, the presence of involuntary contractions occured in 72% of cases and was not associated with worsening of bladder capacity [34]. Rhythmic bowel contractions are triggered by wall distension and it seems to maintain such property after bladder augmentation. Poor results were associated with small reservoir capacity but not with the presence of involuntary contractions [34].
In an impressive large urogenital tuberculosis series with description of 4,298 patients in Russia [23] published in 1997, bladder augmentation with sigmoid was performed in 426 patients since 1960. After describing frequent stenosis of intestine-bladder anastomoses, the authors recommend cystoprostatectomy with orthotopic neobladder with cecum and uretero-ileal anastomosis and invagination of the appendix into the remaining part of the urethra. Due to a lack of data, no conclusion can be made to that proposal.
In conclusion, the aims of surgical treatment of contracted bladders are:
- Quality of life improvement through incontinence treatment and restoration of a reasonable micturitional interval and
- preservation of the upper urinary tract by lowering bladder pressure. Therefore, a low pressure, high capacity reservoir must be created through a bladder augmentation or orthotopic neobladder.
In both cases, the bowel segment used does not affect the result and its detubularization provides higher capacity and compliance of the reservoir, although the cecum may be used successfully with its original tubular configuration. There are no randomized comparative studies between these segments in tuberculosis patients but one retrospective comparative study between detubularized ileocecal and sigmoid segment and non-detubularized sigmoid. Worse results were associated with the non-detubularized sigmoid [34]. The detubularization allows a greater reservoir volume, from 18% to 425% improvement, proportional to the length of the segment to be detubularized and inversely proportional to the radius. The cecum possesses greater radius and volume than the sigmoid and ileum at initial configuration, therefore the volumetric improvement after the detubularization is unnecessary and may explain the good outcome associated with the tubularized cecum [34]. Ureteral reimplantation should be made in cases of stenosis, but is not necessary in reflux [35]. The choice between bladder augmentation and orthotopic neobladder is not well established and there are no comparative studies. Neobladder is advised in very small bladders (less than 15 to 20 ml) or in the presence of pain (suprapubic or perineal) [23], [32], [35]. Pain may not improve after augmentation and may be associated with worse results [34]. No definitive conclusions can be made. The great majority of patients can void spontaneously after surgery and with high volume residual initial attempt of de-obstructive surgery (prostate resection or urethral stenosis surgery) must be done before a self-catheterization regime. Some patients will develop terminal renal failure despite surgery, due to inevitable progression of initial kidney damage. Bladder augmentation allows a better quality of life in patients with some degree of renal failure by improving the micturitional pattern and the augmented bladder may be used to receive a kidney transplant [34].
3.5 Epididymectomy
Surgical treatment of tuberculous epididymitis has declined and lost its importance after tuberculosis pharmacological treatment has been well established [37]. However, there are still three indications for epididymectomy/orchidectomy for tuberculosis:
- Tumor suspicion: in the presence of a solid mass involving scrotal organs, surgical excision through an inguinal incision is necessary to exclude malignancy [38], [39].
- To obtain local control of tuberculous infection: abscess formation, scrotal fistula and persistent local pain may need surgical excision despite pharmacological treatment [40].
- Need of diagnostic improvement: patients with tuberculosis suspicion without pathologic or microbiologic confirmation and with epididymal alterations may be submitted to epididymectomy for diagnostic purpose [38], [39], [40].Surgical treatment can be made as epididymectomy only if the testis is spared from the disease [40].
Surgical treatment can be made as epididymectomy only if the testis is spared from the disease [40].
4 Further research
Regarding surgery treatment of UGTB, the main research interest should be focused on strategies of early diagnosis. Surgical treatment involves correction of chronic lesions due to delayed treatment of this destructive disease. Strategies of screening of UGTB in patients more prone to the disease, such as those with AIDS, immunological deficiency or disease, past history of pulmonary tuberculosis and in those with hematuria, should be performed and established in guidelines after appropriate studies’ results.
5 Conclusions
Many patients of urogenital TB present late with cicatrization sequelae. Multidrug chemotherapy with judicious surgery as and when indicated is the ideal treatment. The results of reconstructive surgery are good and should be done whenever possible. Rigorous and long-term follow‑up is necessary in patients undergoing reconstructive surgery.
References
[1] Krishnamoorthy S, Gopalakrishnan G. Surgical management of renal tuberculosis. Indian J Urol. 2008 Jul;24(3):369-75. DOI: 10.4103/0970-1591.42620[2] Daher Ede F, da Silva GB Jr, Barros EJ. Renal tuberculosis in the modern era. Am J Trop Med Hyg. 2013 Jan;88(1):54-64. DOI: 10.4269/ajtmh.2013.12-0413
[3] Sourial MW, Brimo F, Horn R, Andonian S. Genitourinary tuberculosis in North America: A rare clinical entity. Can Urol Assoc J. 2015 Jul-Aug;9(7-8):E484-9. DOI: 10.5489/cuaj.2643
[4] Goel A, Dalela D. Options in the management of tuberculous ureteric stricture. Indian J Urol. 2008 Jul;24(3):376-81. DOI: 10.4103/0970-1591.42621
[5] Lang EK, Glorioso LW 3rd. Antegrade transluminal dilatation of benign ureteral strictures: long-term results. AJR Am J Roentgenol. 1988 Jan;150(1):131-4. DOI: 10.2214/ajr.150.1.131
[6] Murphy DM, Fallon B, Lane V, O'Flynn JD. Tuberculous stricture of ureter. Urology. 1982 Oct;20(4):382-4. DOI: 10.1016/0090-4295(82)90460-5
[7] Kim SH, Yoon HK, Park JH, Han JK, Han MC, Kim SW, Lee CW. Tuberculous stricture of the urinary tract: antegrade balloon dilation and ureteral stenting. Abdom Imaging. 1993;18(2):186-90. DOI: 10.1007/BF00198060
[8] de la Taille A, Ravery V, Hoffmann P, et al. Le traitement des sténoses de l'uretère par cathéters de dilatation à haute pression [Treatment of ureteral stenosis using high pressure dilatation catheters]. Prog Urol. 1997;7(3):408-414.
[9] Merchant S, Bharati A, Merchant N. Tuberculosis of the genitourinary system-Urinary tract tuberculosis: Renal tuberculosis-Part II. Indian J Radiol Imaging. 2013 Jan;23(1):64-77. DOI: 10.4103/0971-3026.113617
[10] Gupta NP, Kumar R, Mundada OP, Aron M, Hemal AK, Dogra PN, Seth A. Reconstructive surgery for the management of genitourinary tuberculosis: a single center experience. J Urol. 2006 Jun;175(6):2150-4; discussion 2154. DOI: 10.1016/S0022-5347(06)00310-7
[11] Abbara A, Davidson RN; Medscape. Etiology and management of genitourinary tuberculosis. Nat Rev Urol. 2011 Dec 9;8(12):678-88. DOI: 10.1038/nrurol.2011.172
[12] Lee JY, Park HY, Park SY, Lee SW, Moon HS, Kim YT, Lee TY, Park HY. Clinical Characteristics of Genitourinary Tuberculosis during a Recent 10-Year Period in One Center. Korean J Urol. 2011 Mar;52(3):200-5. DOI: 10.4111/kju.2011.52.3.200
[13] Gupta R, Dorairajan LN, Muruganandham K, Manikandan R, Kumar A, Kumar S. Laparoscopic ablative and reconstructive surgeries in genitourinary tuberculosis. JSLS. 2014 Jul-Sep;18(3). pii: e2014.00203. DOI: 10.4293/JSLS.2014.00203
[14] Hemal AK, Gupta NP, Kumar R. Comparison of retroperitoneoscopic nephrectomy with open surgery for tuberculous nonfunctioning kidneys. J Urol. 2000 Jul;164(1):32-5. DOI: 10.1016/S0022-5347(05)67442-3
[15] Zhang X, Zheng T, Ma X, Li HZ, Li LC, Wang SG, Wu ZQ, Pan TJ, Ye ZQ. Comparison of retroperitoneoscopic nephrectomy versus open approaches to nonfunctioning tuberculous kidneys: a report of 44 cases. J Urol. 2005 May;173(5):1586-9. DOI: 10.1097/01.ju.0000154624.44403.b9
[16] Halim A, Gow JG. Genitourinary tuberculosis. In: Husain I, editor. Tropical urology and renal disease. 1st ed. New York: Churchill Livingstone; 1985. p. 118–40.
[17] Chang AH, Blackburn BG, Hsieh MH. Tuberculosis and parasitic infections of the genitourinary tract. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 11th ed. Philadelphia: Elsevier; 2016. p. 421–46.
[18] Flechner SM, Gow JG. Role of nephrectomy in the treatment of non-functioning or very poorly functioning unilateral tuberculous kidney. J Urol. 1980 Jun;123(6):822-5. DOI: 10.1016/S0022-5347(17)56149-2
[19] Figueiredo AA, Lucon AM. Urogenital tuberculosis: update and review of 8961 cases from the world literature. Rev Urol. 2008 Summer;10(3):207-17.
[20] Bansal P, Bansal N. The surgical management of urogenital tuberculosis our experience and long-term follow-up. Urol Ann. 2015 Jan-Mar;7(1):49-52. DOI: 10.4103/0974-7796.148606
[21] Cek M, Lenk S, Naber KG, Bishop MC, Johansen TE, Botto H, Grabe M, Lobel B, Redorta JP, Tenke P; Members of the Urinary Tract Infection (UTI) Working Group of the European Association of Urology (EAU) Guidelines Office. EAU guidelines for the management of genitourinary tuberculosis. Eur Urol. 2005 Sep;48(3):353-62. DOI: 10.1016/j.eururo.2005.03.008
[22] Tian X, Wang M, Niu Y, Zhang J, Song L, Xing N. Retroperitoneal Laparoscopic Nephroureterectomy for Tuberculous Nonfunctioning Kidneys: a single-center experience. Int Braz J Urol. 2015 Mar-Apr;41(2):296-303. DOI: 10.1590/S1677-5538.IBJU.2015.02.16
[23] Figueiredo AA, Lucon AM, Junior RF, Srougi M. Epidemiology of urogenital tuberculosis worldwide. Int J Urol. 2008 Sep;15(9):827-32. DOI: 10.1111/j.1442-2042.2008.02099.x
[24] Zuban' ON, Volkov AA, Sushchiĭ EA, Murav'ev AN. [Surgical urinary and male genital tuberculosis]. Probl Tuberk Bolezn Legk. 2008;(12):57-60.
[25] Figueiredo AA, Lucon AM, Arvellos AN, Ramos CO, Toledo AC, Falci R Jr, Gomes CM, Recaverren FE, Netto JM, Srougi M. A better understanding of urogenital tuberculosis pathophysiology based on radiological findings. Eur J Radiol. 2010 Nov;76(2):246-57. DOI: 10.1016/j.ejrad.2009.05.049
[26] Kerr WK, Gale GL, Peterson KS. Reconstructive surgery for genitourinary tuberculosis. J Urol. 1969 Mar;101(3):254-66. DOI: 10.1016/S0022-5347(17)62324-3
[27] Wesolowski S. Late results of cystoplasty in chronic tuberculous cystitis. Br J Urol. 1970 Dec;42(6):697-703. DOI: 10.1111/j.1464-410X.1970.tb06794.x
[28] Abel BJ, Gow JG. Results of caecocystoplasty for tuberculous bladder contracture. Br J Urol. 1978 Dec;50(7):511-6. DOI: 10.1111/j.1464-410X.1978.tb06202.x
[29] Dounis A, Gow JG. Bladder augmentation--a long-term review. Br J Urol. 1979 Aug;51(4):264-8. DOI: 10.1111/j.1464-410X.1979.tb04706.x
[30] Lunghi F, Nicita G, Selli C, Rizzo M. Clinical aspects of augmentation enterocystoplasties. Eur Urol. 1984;10(3):159-63. DOI: 10.1159/000463779
[31] Benchekroun A, Hachimi M, Marzouk M, Lakrissa A, Abakka T, Essakalli N, Faik M, Bezad R. [Enterocystoplasty for small tuberculous bladder. Apropos of 40 cases]. Acta Urol Belg. 1987;55(4):583-90.
[32] Hemal AK, Aron M. Orthotopic neobladder in management of tubercular thimble bladders: initial experience and long-term results. Urology. 1999 Feb;53(2):298-301. DOI: 10.1016/S0090-4295(98)00504-4
[33] Aron M, Gupta NP, Hemal AK, Seth A, Dogra PN, Wadhwa SN. Surgical management of advanced bladder tuberculosis. Aust N Z J Surg. 2000;70:A20.
[34] de Figueiredo AA, Lucon AM, Srougi M. Bladder augmentation for the treatment of chronic tuberculous cystitis. Clinical and urodynamic evaluation of 25 patients after long term follow-up. Neurourol Urodyn. 2006;25(5):433-40. DOI: 10.1002/nau.20264
[35] Singh V, Sinha RJ, Sankhwar SN, Sinha SM. Reconstructive surgery for tuberculous contracted bladder: experience of a center in northern India. Int Urol Nephrol. 2011 Jun;43(2):423-30. DOI: 10.1007/s11255-010-9815-7
[36] Kholtobin DP, Kul'chavenia EV, Khomiakov VT. [Bladder tuberculosis stage 4: how to restore urination?]. Urologiia. 2014 Sep-Oct;(5):26-9.
[37] Mochalova TP, Starikov IY. Reconstructive surgery for treatment of urogenital tuberculosis: 30 years of observation. World J Surg. 1997 Jun;21(5):511-5. DOI: 10.1007/PL00012278
[38] Badmos KB. Tuberculous epididymo-orchitis mimicking a testicular tumour: a case report. Afr Health Sci. 2012 Sep;12(3):395-7.
[39] Kulchavenya E, Kim CS, Bulanova O, Zhukova I. Male genital tuberculosis: epidemiology and diagnostic. World J Urol. 2012 Feb;30(1):15-21. DOI: 10.1007/s00345-011-0695-y
[40] Gómez García I, Gómez Mampaso E, Burgos Revilla J, Molina MR, Sampietro Crespo A, Buitrago LA, Gómez Rodríguez A, Baquero F. Tuberculous orchiepididymitis during 1978-2003 period: review of 34 cases and role of 16S rRNA amplification. Urology. 2010 Oct;76(4):776-81. DOI: 10.1016/j.urology.2010.01.033




