Urogenital tuberculosis as a part of extrapulmonary tuberculosis in two epidemic regions
Mahmadzarif Merganov 2
1 Novosibirsk Research TB Institute, Novosibirsk State Medical University, Novosibirsk, Russia
2 National Center of Tuberculosis, Pulmonary Diseases and Thoracic Surgery, Dushanbe, Republic of Tajikistan
Abstract
Introduction: The role of extrapulmonary tuberculosis (EPTB) in general and urogenital tuberculosis (UGTB) in particular is under-estimated in official statistics, medical practice and by the population.
The purpose of this report was to estimate the relative importance of UGTB in the spectrum of EPTB in two epidemic regions of central Asia (Siberia, Russian Federation, and the Republic of Tajikistan) and its relation to human immunodeficiency virus (HIV) comorbidity, age and sex.
Material and Methods: A retrospective descriptive report based on official statistics from the tuberculosis dispensaries (special outpatient clinics) of Siberia and Tajikistan for 2018 was made.
Results: In Siberia the most common form was bone and joint tuberculosis (BJTB) – 43.6%, followed by urogenital tuberculosis (UGTB) – 21.3%, and tuberculosis of the central nervous system (CNS-TB) – 17.0%. In Tajikistan the leading localization was also BJTB (46.0%), followed by tuberculosis of peripheral lymph nodes (LNTB) – 27.7%, whereas the percentage of UGTB was only 6.1%. Patients combining EPTB and HIV infection were 36.8% in Siberia but only 3.5% in Tajikistan. CNS-TB was the predominating localization in patients with HIV infection in Siberia (38.3%), while in Tajikistan LNTB and BJTB (37.9% and 31.0%, respectively) were the most common sites. UGTB was the rarest form among HIV-infected patients reported in both regions.
In Siberia CNS-TB was detected twice as often in men than in women (67.9% versus 32.1%) and a similar proportion was described for BJTB (62.9% versus 37.1% ). In Tajikistan female patients predominated among CNS-TB (71.4%), LNTB (81.5%), and abdominal tuberculosis (68.9%). UGTB equally often affected men and women in both regions.
Conclusions: HIV-comorbidity significantly changes the spectrum of EPTB. UGTB had a negative correlation with HIV infection. Spectrum of EPTB is variable from region to region. The different sex-age relationships with EPTB in the two regions cannot be explained.
Summary of findings
- It is difficult to obtain accurate figures on the spectrum of extrapulmonary tuberculosis (EPTB) in the literature. This is mainly due to the lack of uniform terminology and of established definitions of extrapulmonary localization.
- Statistical analysis of EPTB is therefore more complicated than pulmonary tuberculosis.
- There are huge differences in the incidence rate even in neighboring regions, which may reflect weaknesses in data collection, the lack of skilled specialists and a limited level of suspicion in general practice.
- Bone and joint tuberculosis, was the most common form of EPTB in both Siberia and Tajikistan. UGTB was the third most common form in Siberia, and next to last in Tajikistan.
- Concomitant HIV infection remarkably changed the spectrum of EPTB. In Siberia 38.6% of patients with EPTB had an HIV infection, and 38.3% of them had CNS-TB, whereas in Tajikistan only 3.5% of patients with EBTB were infected with HIV. A concomitant HIV infection significantly delays diagnosis and is changing both the clinical presentation and the histological aspect of EPTB.
- The lack of accurate statistics over many years makes it difficult to estimate a reasonably correct level of the epidemic situation of EPTB. An Analysis of the sex-age structure of the most common forms of EPTB also revealed significant differences between the two areas.
1 Introduction
The World Health Organization (WHO) emphasized that a representative incidence of tuberculosis at national level is difficult to obtain because this would require long-term studies in large cohorts (hundreds of thousands) at high costs and challenging logistics [1]. Extrapulmonary tuberculosis (EPTB) is even more difficult for statistical measurement and control. Pulmonary tuberculosis is the most common form of all TB localizations and a priority for the national public health programs. However, EPTB is an important issue because of substantial morbidity and mortality [2].
The role of EPTB is considered as underreported in general medical practice and official statistic and poorly recognized in the population. The low index of suspicion of EPTB [3] results in delayed diagnosis, especially in case of poor therapeutic reserves and surgery options. Inefficiency of antibacterial therapy for urogenital infections may be explained by the resistance of the uropathogen while, actually, this UTI might be a mask of UGTB – or comorbidity with a UGTB disease [4]. EPTB has no specific symptoms and the presence of mycobacteria in urine may be very limited. In some forms of EPTB it might even be impossible to identify M. tuberculosis before surgery (e.g. tuberculosis of the adrenal gland, spleen, pericardium). In some situations, the risk of obtaining tissue for investigations may even exceed the possible benefits, due to the threat of a fulminant generalization of the mycobacterial infection.
EPTB diagnosis is often delayed and obtained first after general surgery following an urgent situation (e.g. bleeding, abscess). A concomitant human immunodeficiency virus (HIV) infection significantly delays diagnosis further and changes both the clinical presentation and the histological aspect of EPTB [3].
Official statistics do not account for EPTB in patients with pulmonary tuberculosis. Therefore, we do not see the true epidemiological spectrum of EPTB. Tuberculosis is a systemic infection and it is believed that the eradication of M. tuberculosis results in a cure from the disease. However, extrapulmonary forms cannot be neglected because they affect different organs and systems that will require a differentiated approach to treatment.
The purpose of this descriptive retrospective study was to estimate the place of UGTB in the spectrum of EPTB in two high burden epidemic regions, Siberia in the Russian Federation and the Republic of Tajikistan, and to evaluate the roles of HIV infection, sex and age.
2 Methods
2.1 Setting and study population
The Novosibirsk Research TB Institute provides monitoring of anti-TB service in Siberia and Far East, i.e. the Asian part of the Russian Federation from the Ural Mountains to the Pacific Ocean. This extensive geographic area, called Siberia in the present study, is divided into 21 regions. The epidemic situation of TB is higher in Siberia than in the other regions of the Russian Federation: In 2018 the incidence rate was 72.8 per 100,000 population compared to 44.4 per 100,000 inhabitants in the Russian Federation overall.
The National Center of Tuberculosis, Pulmonary Diseases and Thoracic Surgery in Dushanbe provides the same surveillance in the Republic of Tajikistan. The incidence in Tajikistan was 56.6 per 100,000 inhabitants in 2018. The disease burden of tuberculosis is high in both the Russian Federation and the Republic of Tajikistan [1].
2.2 Inclusion criteria
In this analysis we included all patients with active newly-diagnosed or relapsing EPTB reported between 1 January 2018 and 31 December 2018 in the official statistics of the tuberculosis dispensaries of Siberia and Tajikistan, considering sex, age and concomitant HIV infection. EPTB was confirmed by positive cultures and/or histological specimens or by strong clinical evidence consistent with active EPTB, in accordance with WHO guidelines. EPTB was defined as tuberculosis affecting any organ or tissue, outside the respiratory system (lungs, pleura, bronchial three, intrathoracic lymph nodes). The HIV status was verified in all EPTB patients.
2.3 Exclusion criteria
Pulmonary tuberculosis only was considered as an exclusion criterion.
2.4 Design of study
A simple cohort, open, retrospective study was performed. Correlation of the EPTB spectrum with sex and age, HIV infection was calculated.
2.5 Statistical analysis
Statistical analysis was performed using Person’s chi-square and Fisher’s exact test to compare independent proportions. Statistically, p <0.05 was considered as significantly different.
2.6 Ethics
This study was approved by the Ethic Committee of Novosibirsk Research TB Institute as a quality assurance project. All data were retrieved from official reports and written informed consent was not considered necessary for this study.
3 Results
3.1 Total number of patients with EPTB
Between 1 January and 31 December 2018, a total of 582 patients were diagnosed in Siberia with extrapulmonary tuberculosis of whom 508 (87.3%) were new cases and 74 (12.7%) were relapsing disease (table 1). The corresponding total number in Tajikistan was 819 (table 1) without differentiating new and relapse infection, respectively.
|
Localization |
HIV negative |
HIV positive |
Totals
|
p-value* |
|||
|
|
n |
% |
n |
% |
n |
% |
|
|
CNS-TB |
17 |
4.6 |
82 |
38.3 |
99 |
17.0 |
0.002 |
|
UroTB |
81 |
22.0 |
10 |
4.7 |
91 |
15.6 |
0.003 |
|
FGTB |
32 |
8.7 |
1 |
0.5 |
33 |
5.7 |
0.001 |
|
UGTB total |
113 |
30.7 |
11 |
5.2 |
124 |
21.3 |
0.003 |
|
BJTB |
168 |
45.6 |
86 |
40.2 |
254 |
43.6 |
0.08 |
|
LNTB |
49 |
13.3 |
24 |
10.2 |
68 |
11.7 |
0.06 |
|
Others |
21 |
5.8 |
19 |
8.9 |
37 |
6.4 |
0.04 |
|
Totals |
368 |
63.2 |
181 |
36.8 |
582 |
100 |
0.004 |
*p<0.005 – significant difference between HIV negative and HIV positive
CNS-TB – tuberculosis of central nervous system, uroTB – tuberculosis of urinary system and male genitals (urological tuberculosis), FGTB – female genital tuberculosis, UGTB – urogenital tuberculosis, BJTB – Bone and joints tuberculosis, LNTB – tuberculosis of peripheral lymph nodes.
3.2 Anatomic localization profile
Bone and joint tuberculosis was the most common extrapulmonary site in both regions. The distribution of EPTB for Siberia is given in figure 1. However, the distribution of Tajikistan showed noticeable differences (table 1; figure 1). Urogenital tuberculosis was diagnosed in 124/582 (21.3%) patients in Siberia compared with only 50 (6.1%) in Tajikistan (p <0.05). On the opposite, lymph node tuberculosis was diagnosed in Tajikistan more than two times the rate in Siberia (27.7 versus 11.7%), p <0.05.
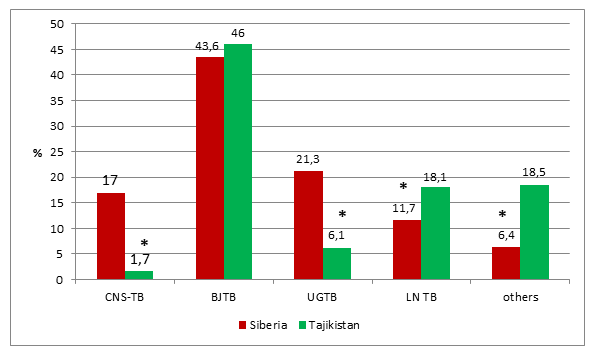
*p <0.05
BJTB–Bone and joints tuberculosis, UGTB–urogenital tuberculosis, CNS-TB –tuberculosis of central nervous system, LNTB–tuberculosis of peripheral lymph nodes
3.3 EPTB and HIV co-infection
HIV status was verified in all patients. HIV co-infection was diagnosed in 214/582 (36.8%) patients in Siberia, but only in 29/819 (3.5%) in Tajikistan. The distribution of extrapulmonary localization in both regions is given in table 1, showing that BJTB was the main site, followed by CNS-TB in Siberia. In contrast, in Tajikistan LNTB was the predominating localization, closely followed by BJTB. CNS-TB was noticeably lower in Tajikistan compared with Siberia. UGTB was less frequent in HIV-positive patients compared to HIV-negative patients.
Immunocompetent patients had mostly newly diagnosed EPTB (88.9% – 327 patients), while 11.1% (41 patients) had a relapse of tuberculosis (p <0.001). Immunocompromised patients (84.6% – 181 patients) had newly diagnosed EPTB, while 15.4% (33 patients) had a relapse of tuberculosis (p <0.001). Thus, the difference between frequency of newly diagnosed EPTB and relapse in both groups was significant (5.5-fold), but this proportion was the same in both the groups of HIV-positive and HIV-negative patients (p >0.05).
When we considered EPTB as a whole, independent of HIV status, the leading localization of EPTB in Siberia in 2018 was BJTB (254 patients – 43.6%); UGTB was the second most common form (124 patients – 21.3%), followed by CNS-TB (99 patients – 17.0%).
The classification of EPTB into HIV-positive and HIV-negative patients resulted in significant differences. This is demonstrated in table 1.
Among HIV-positive EPTB patients in Siberia, the most common form was CNS-TB (38.3%); this form of EPTB was diagnosed in HIV-infected patients 8.3 times more often than in immunocompetent patients. UGTB demonstrated an inverse correlation. UroTB (tuberculosis of the urinary system and male genital organs) developed in HIV-positive patients 4.7 times less often than among HIV-negative patients. With tuberculosis of the female genital organs, this proportion reached a 17-fold magnitude. UGTB as a whole (urinary system and male and female genitals) was about 6 times less common in HIV-infected patients than in HIV-negative patients. Other localizations did not show such impressive variations, although both BJTB and LNTB were also found by 5.4%–3.1% less often in HIV-infected patients. We have no convincing explanation for this imbalance.
Only 29 EPTB patients (3.5%) in Tajikistan were HIV positive; this figure is ten times less than in Siberia. Among HIV-positive patients, LNTB (37.9%) and BJTB (31.0%) predominated followed by abdominal tuberculosis (17.2%). In Tajikistan CNS-TB in HIV-positive patients was diagnosed in 6.9% only, 5.5 times less often than in Siberia. Like in Siberia, in Tajikistan UGTB was the last in the list of EPTB localizations in co-infected patients with a comorbidity of HIV infection in 3.4% of cases only.
3.4 EPTB and sex
CNS-TB was found twice as often in men than in women (67.9% versus 32.1%) in Siberia (p <0.03). BJTB had about the same proportion: 62.9% male patients and 37.1% female patients (p <0.03). Like in Tajikistan, the sex proportion of UGTB patients in Siberia was male/female = 0.9. On the contrary, LNTB in Siberia was diagnosed in men more often than in women (55.7%; p >0.05). Here again, we came across tendencies which we cannot explain.
CNS-TB was a ’female’ disease in Tajikistan: 71.4% of the patients with CNS-TB were females. On the contrary, BJTB was more often diagnosed in male patients (53.9%). UGTB affected men and women equally with a male/female ratio of 0.9. LNTB (81.5%) as well as abdominal tuberculosis (68.9%) were more frequent in females in Tajikistan (p <0.05).
3.5 Sex-age structure
The Analysis of the sex-age structure of the most common forms of EPTB in both regions revealed significant discrepancies. Figures 2–7 demonstrate sex-age characteristics of patients with BJTB and UGTB, and LNTB in Siberia and in Tajikistan.
In Siberia the BJTB incidence was maximal in patients aged 35–44 years, and men prevailed twice as often as women (p <0.001; figure 2).
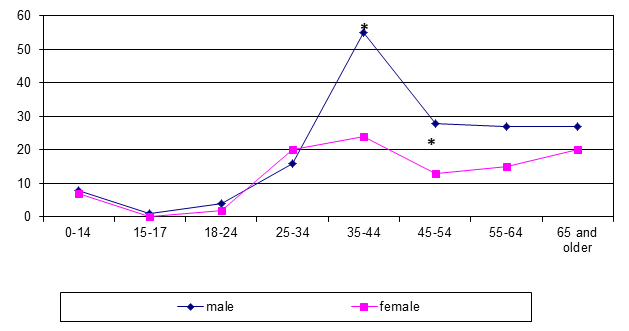
*p <0.05
In Tajikistan, the majority of patients fell ill with BJTB between the ages of 25 and 34, and women were infected more often than men (p <0.005; figure 3).
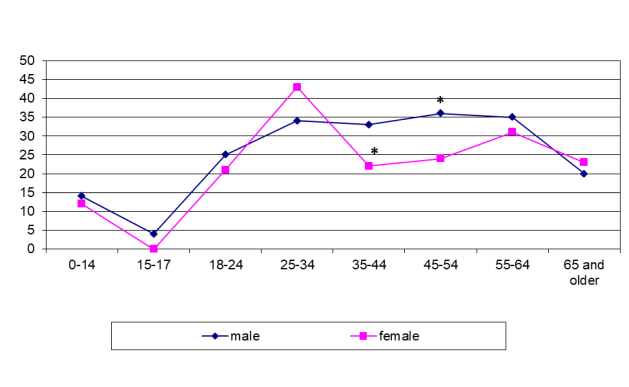
*p <0.05
In both regions the peak of incidence of UGTB in females aged 25–34 years (p <0.0001) was probably due to the contribution of female genital tuberculosis. Another logical explanation could not be found (figure 4 and figure 5).
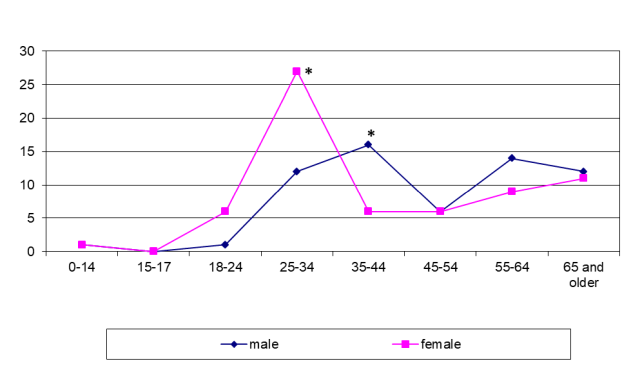
*p <0.05
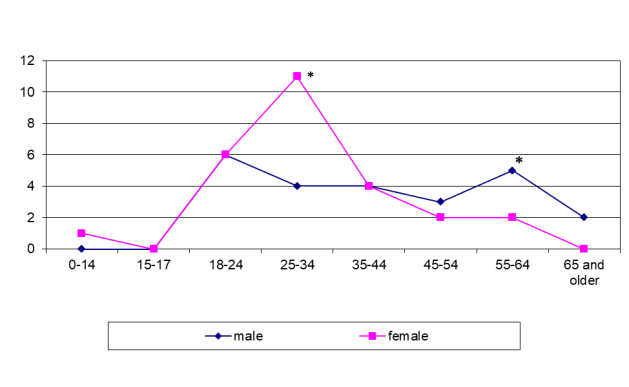
*p <0.05
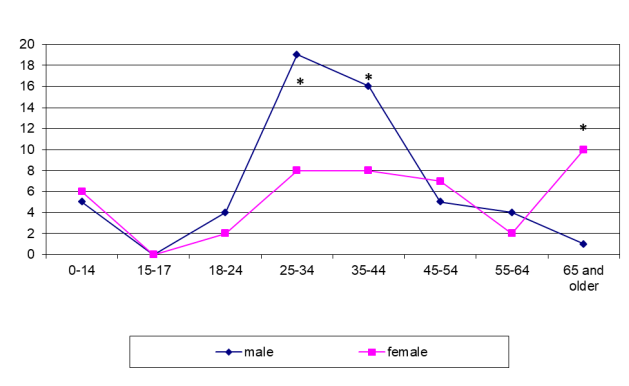
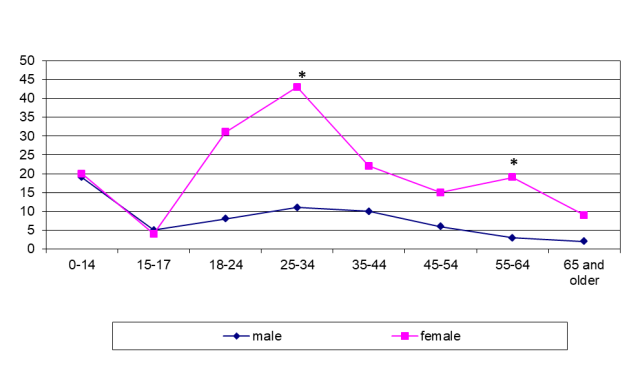
Even more striking differences were found in the sex-age structure of patients with LNTB. LNTB was seen in Siberia mostly in men of 25–44 years while it was diagnosed in the younger range (18–34 years) in females in Tajikistan (figure 6 and figure 7). In some cases the patients presented late with already established fistulous forms.
*p <0.05
*p <0.05
Even larger differences were found in the sex-age structure of patients with LNTB. In Siberia, LNTB was seen mostly in men of 25–44 years (p <0.001; figure 6) while it was diagnosed in the younger range (18–34 years) in females in Tajikistan (p <0.001; figure 7). In some cases, the patients presented late with already established fistulous forms.
LNTB is not very difficult to diagnose. Open biopsy may be performed, and a pathomorphological and microbiological investigation of tissue may confirm the diagnosis. Unfortunately, even now some patients do not see their doctor until complications (fistulous forms) have developed.
Comparative age characteristic of EPTB patients as a whole in the two epidemic regions are shown in figure 8.
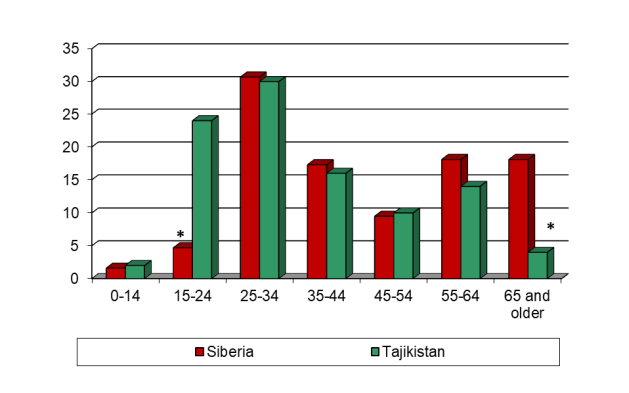
*p <0.05
4 Discussion
The analysis and interpretation of figures on the distribution of EPTB found in the literature is difficult for several reasons. This is mainly due to a lack of uniform terminology, definitions and classification of extrapulmonary tuberculosis. For example, LN-TB was diagnosed in half of the EPTB patients in Bangladesh [5], but no information was given as to which lymph nodes were involved. Tuberculosis of the intrathoracic lymph nodes and tuberculous mesadenitis are special localizations that must be identified as such. In some countries tuberculosis of the pleura and intrathoracic lymph nodes are classified as extrapulmonary localization; in others, primarily in the Russian Federation and Belarus, they are considered as respiratory tuberculosis [6], [7].
Even in the same region the EPTB spectrum varies significantly with the period of time. From 2006 to 2016, in Siberia, the proportion of CNS-TB doubled, while the proportion of UGTB decreased almost twofold [8], [9]. Many authors assessed the total EPTB spectrum for a very long period, up to 10–20 years [10], [11]. In Siberia, we see that this is incorrect as there is a big difference from year to year. In Saudi Arabia from 2015 to 2019 the proportion of LN-TB (of unknown localization) increased by 13.6%, and the proportion of UGTB increased 4 times [12], [13].
In 2018 the total number of reported tuberculosis cases in our two regions was 19,903 for Siberia and 5102 for the Republic of Tajikistan. The EPTB cases accounted for in the present review were 582 for Siberia and 819 for Tajikistan.
The smallest proportion of pleural tuberculosis (3.4%) was recorded in Australia in 2008–2015, the highest proportion (47.4%) was seen in South Korea in 2015 [14], [15]. The minimal rate of UGTB was 1.1% in Saudi Arabia in 2015, and the highest (35.0%) was seen in Siberia in 2006 [8], [13]. The smallest proportion of BJTB (5% in each country) was in Spain (2013–2016), Brazil (2007–2011) and Saudi Arabia (2019) [12], [16], [17]. CNS-TB was diagnosed least often in Poland in 2013 (1.7%) and most often in Siberia in 2016–2017 (18.7%) [18], [19].
In countries where abdominal tuberculosis is registered separately, this localization occurs with a frequency of 6% (Ghana, 2010–2013) up to 18.7% (Saudi Arabia, 2019) [12], [20]. In Australia pericardial tuberculosis (3.4%), skin tuberculosis (5%) and eye tuberculosis (2.6%) were diagnosed unexpectedly often [15]. Eye tuberculosis was 2% of the total EPTB spectrum in Brazil (2007–2011) [16]. In Siberia eye tuberculosis accounted for 7.4% in 1999 and in 2008 its proportion decreased to 4.4%. Since 2009, eye tuberculosis is regarded as an independent type of EPTB and is not taken into account by official EPTB statistics in the Russian Federation [8], [9].
A large disproportion was also revealed in the age spectrum of patients. A comparison of data from Siberia, Tajikistan and Germany has shown that in developed countries UGTB is mainly diagnosed in patients with older age, and in epidemic regions in younger ones [21], [22].
Probably, in epidemic regions UGTB is more evident and there is a higher level of suspicion. Also, a lower life expectancy may explain a higher incidence rate in young people.
5 Further research
It is necessary to continue and extend studies on epidemiology of UGTB. UGTB should be considered as a part of EPTB. We need a unique classification and terminology of UGTB, which should be approved by all medical communities. A cheap and effective screening of UGTB is recommended. A good screening and a high level of suspicion will increase in-time diagnosis of UGTB.
6 Conclusion
The incompleteness of statistics (the use of various classifications of EPTB, analysis of total data over too long time-periods) does not allow for a correct assessment of the epidemic situation on EPTB. The Analysis of the sex-age structure of the most common forms of EPTB also revealed significant discrepancies. The spectrum of EPTB is different even in neighboring regions. Concomitant HIV infection has a big influence on the spectrum of EPTB. UGTB has a negative correlation with HIV infection.
7 Acknowledgement
The authors would like to thank doctors from tuberculosis dispensaries in Siberia and Tajikistan who filled in and submitted special forms on EPTB patients.
8 Conflict of interest
Both authors declare that they have no competing interests. There were no grants for this study.
References
[1] World Health Organization. Global tuberculosis report 2017. WHO: Geneva; 2017. Available from: http://apps.who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf?ua=1[2] Qian X, Nguyen DT, Lyu J, Albers AE, Bi X, Graviss EA. Risk factors for extrapulmonary dissemination of tuberculosis and associated mortality during treatment for extrapulmonary tuberculosis. Emerg Microbes Infect. 2018 Jun;7(1):102. DOI: 10.1038/s41426-018-0106-1
[3] Kulchavenya E, Naber K, Bjerklund Johansen TE. Urogenital tuberculosis: classification, diagnosis, and treatment. European Urology Supplement. 2016;15(4):112-21. DOI: 10.1016/j.eursup.2016.04.001
[4] Wagenlehner F, Tandogdu Z, Bartoletti R, et al. The global prevalence of infections in urology study: a long-term, worldwide surveillance study on urological infections. Pathogens. 2016;5(1):10. Published 2016 Jan 19. DOI: 10.3390/pathogens5010010
[5] Quddus MA, Uddin MJ, Bhuiyan MM. Evaluation of extra pulmonary tuberculosis in Bangladeshi patients. Mymensingh Med J. 2014;23(4):758-63.
[6] Tuberkulez v Rossiyskoy Federatsii 2012, 2013, 2014 g. Analiticheskiy obzor statisticheskikh pokazateley, ispolzuemykh v Rossiyskoy Federatsii i v mire [Tuberculosis in the Russian Federation in 2012, 2013. 2014. Analytic review of statistic rates used in the Russian Federation and in the world]. Moscow; 2015.
[7] Solonko II, Gurevich GL, Skryagina ЕМ, Dusmikeeva МI. Extrapulmonary tuberculosis: clinical-epidemiologacal characteristic and diagnostic. Tuberculez I bolezni legkih. 2018;96(9):22-8. DOI: 10.21292/2075-1230-2018-96-6-22-28
[8] Kulchavenya E, Brizhatyuk Е, Коveshnikova Е. New tendencies in epidemic situation on tuberculosis of extra-thoracal localizations in Siberia and Far East. Tuberculez I bolezni legkih. 2009;86(10):27-31.
[9] Kulchavenya E, Zhukova I, Kholtobin D. Spectrum of urogenital tuberculosis. J Infect Chemother. 2013 Jan;19(5):880-3. DOI: 10.1007/s10156-013-0586-9
[10] Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009 Nov;49(9):1350-7. DOI: 10.1086/605559
[11] Gonzales OY, Adams G, Teeter LD, Bui TT, Musser SM, Gravis EA. Extra-pulmonary manifestations in a large metropolitan area with a low incidence of tuberculosis. Int J Tuberc Lung Dis. 2003;7(12):1178-85.
[12] Al-Hajoj S, Shoukri M, Memish Z, AlHakeem R, AlRabiah F, Varghese B. Exploring the sociodemographic and clinical features of extrapulmonary tuberculosis in Saudi Arabia. PLoS One. 2015;10(2):e0101667. Published 2015 Feb 3. DOI: 10.1371/journal.pone.0101667
[13] Al-Ghafli H, Varghese B, Enani M, Alrajhi A, Al Johani S, Albarrak A, Althawadi S, Elkizzi N, Al Hajoj S. Demographic risk factors for extra-pulmonary tuberculosis among adolescents and adults in Saudi Arabia. PLoS One. 2019 Mar 27;14(3):e0213846. DOI: 10.1371/journal.pone.0213846
[14] Pollett S, Banner P, O'Sullivan MV, Ralph AP. Epidemiology, diagnosis and management of extra-pulmonary tuberculosis in a low-prevalence country: a four year retrospective study in an Australian tertiary infectious diseases unit. PLoS ONE. 2016;11(3):e0149372. DOI: 10.1371/journal.pone.0149372
[15] Lee HY, Lee J, Lee YS, Kim MY, Lee HK, Lee YM, Shin JH, Ko Y. Drug-resistance pattern of Mycobacterium tuberculosis strains from patients with pulmonary and extrapulmonary tuberculosis during 2006 to 2013 in a Korean tertiary medical center. Korean J Intern Med. 2015 May;30(3):325-34. DOI: 10.3904/kjim.2015.30.3.325
[16] Gomes T, Reis-Santos B, Bertolde A, Johnson JL, Riley LW, Maciel EL. Epidemiology of extrapulmonary tuberculosis in Brazil: a hierarchical model. BMC Infect Dis. 2014 Jan;14:9. DOI: 10.1186/1471-2334-14-9
[17] Arnedo-Pena A, Romeu-Garcia MA, Meseguer-Ferrer N, Vivas-Fornas I, Vizcaino-Batllés A, Safont-Adsuara L, Bellido-Blasco JB, Moreno-Muñoz R. Pulmonary versus extrapulmonary tuberculosis associated factors: a case-case study. Microbiol Insights. 2019;12:1178636119840362. DOI: 10.1177/1178636119840362
[18] Korzeniewska-Koseła M. Tuberculosis in Poland in 2013. Przegl Epidemiol. 2015;69(2):277-82, 389-93.
[19] Kulchavenya E, Naber K Johansen TEB. Influence of HIV Infection on Spectrum of Extrapulmonary Tuberculosis. J Infect Dis Ther. 2018;6:377. DOI: 10.4172/2332-0877.1000377
[20] Ohene SA, Bakker MI, Ojo J, Toonstra A, Awudi D, Klatser P. Extra-pulmonary tuberculosis: a retrospective study of patients in Accra, Ghana. PLoS ONE. 2019;14(1):e0209650. DOI: 10.1371/journal.pone.0209650
[21] Ducomble T, Tolksdorf K, Karagiannis I, et al. The burden of extrapulmonary and meningitis tuberculosis: an investigation of national surveillance data, Germany, 2002 to 2009. Euro Surveill. 2013;18(12):20436. Published 2013 Mar 21.
[22] Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Respir J. 2008 Jan;31(1):99-105. DOI: 10.1183/09031936.00020607




