[The relevance of temporal coding for spatial hearing with cochlear implants]
Nicole Rosskothen-Kuhl 1,2Jan W. H. Schnupp 3,4
1 Neurobiological Research Laboratory, Section for Experimental and Clinical Otology, Medical Center – University of Freiburg, Faculty of Medicine, Freiburg, Germany
2 Bernstein Center Freiburg & Faculty of Biology, University of Freiburg, Germany
3 Gerald Choa Neuroscience Institute, The Chinese University of Hong Kong, Sha Tin, China
4 Department of Otorhinolaryngology, Head and Neck Surgery, The Chinese University of Hong Kong, Sha Tin, China
Abstract
Although cochlear implants (CIs) have revolutionized hearing restoration, they still exhibit limitations. While efforts have focused on optimizing the delivery of spectral information, relatively little attention has been paid to replicating the precise temporal firing patterns of the auditory nerve observed in normal hearing. Our research explores factors contributing to the poor spatial hearing with CIs by investigating the sensitivity to interaural time differences (ITDs) in cochlear implanted rats. The use of this animal model allows us to circumvent confounds arising from patient needs and clinical practice that plague research on human volunteers. We trained and tested neonatally deafened rats with bilateral CIs delivering exclusively informative pulse timing ITDs throughout, and found that their ITD discrimination thresholds were as good as those of normally hearing individuals even at clinical stimulation rates. Furthermore, we observed that delivering ITDs via pulse timing is much more effective than delivering ITDs via pulse train envelopes, and that even small conflicting ITDs can confound sizeable interaural level differences (ILDs). Our findings thus demonstrate that much better outcomes with bilateral CIs should be achievable in principle if patients were given useful and precise pulse timing cues throughout. Our results indicate that uninformative pulse timing delivered by the majority of current clinical CI processors may hinder spatial hearing in CI patients.
Keywords
deafness, prosthetics, cochlear implant, binaural hearing, interaural time difference, psychoacoustics, hearing experience
Introduction
At a fundamental level, the sense of hearing concerns itself almost exclusively with time and timing, and our auditory system appears to be far better at measuring and processing temporal relationships of events than any of our other senses. Sound waves are minuscule but extremely rapid fluctuations in air pressure. The air pressure change per se is so tiny as to be completely inconsequential. Although sound transmits very little mechanical energy, our hearing is astonishingly precise at decoding the temporal patterns of these tiny pressure fluctuations, providing us with valuable information about our environment.
The inner ear makes a significant contribution to this temporal pattern analysis by performing a time-frequency analysis while the incoming sound waves are passed through the mechanical filter bank of the cochlea. This establishes a place code for the spectral content of sound in the auditory nerve (AN), but it also generates a time code, as the action potentials of the auditory nerve fibers synchronize with the temporal patterns in each frequency band with microsecond accuracy. Cochlear implant (CI) research primarily attempted to optimize the transmission of place coded information to the AN, for example by seeking to reduce interactions between neighboring electrode channels through measures such as interleaved sampling [1] or current focusing [2], [3]. However, relatively little effort has been expended to replicate the extraordinarily precise temporal firing patterns that can be observed when the normally developed auditory nerve encodes natural sounds [4]. The majority of current clinical CI processors offer pulsatile electrical stimulation with a fixed pulse rate between 900 and 3,700 pulses per second (pps) on most or all of their stimulation channels, irrespective of the temporal fine structure of the sound waves to be encoded [5], [6], [7], [8]. The question we address here is whether or to what extent this neglect of temporal fine structure coding in current stimulation strategies represents a missed opportunity for optimal hearing perception in CI patients.
In our recent work, we sought to address this question in a series of behavioral and electrophysiological experiments investigating the ability of laboratory rats to resolve tiny interaural time differences (ITDs). The normally hearing auditory system is capable of resolving ITD of a few tens of microseconds and uses this as a powerful cue for sound source lateralization [9], [10] and auditory scene analysis [11]. Furthermore, it has been suggested that ITD discrimination and the ability of the auditory system to use temporal cues for the arguably even more important pitch perception skills may rely on common, underlying temporal processing mechanisms in the auditory brainstem [12]. ITD discrimination is therefore not only a remarkable and helpful ability, but can also be a useful indicator of the general competence of an auditory system to process temporal cues in the sub-millisecond range. At the same time, ITD discrimination is relatively easy to measure experimentally in both human and animal subjects.
ITD sensitivity in human bilateral CI patients
At present, ITD sensitivity is generally substantially worse in human bilateral CI (biCI) patients than normally hearing listeners. With the exception of the FSP/FS4/FS4p fine structure strategy from MED-EL, which we will discuss in more detail at the end of this article, standard clinical practice is to provide patients with two independent processors that deliver electrical pulses at fixed pulse rates and at times that are independent of incoming sounds or the timing of pulses in the other ear. Interestingly, however, the ITD sensitivity of CI patients remains poor even when tested with experimental processors that deliver precise pulse times. Patients with early acquired hearing loss show the poorest ITD sensitivity. This is illustrated, for example, by Litovsky et al. [13], who reviewed data from a cohort of 34 bilateral CI patients with different onset times of deafness. We replotted their data here in Figure 1 [Fig. 1]. All symbols in Figure 1 [Fig. 1] show behavioral ITD sensitivity thresholds measured with electrical pulse train stimuli delivered to electrodes in the middle turn of the cochlea of each ear. Experimental processors were used that enable the delivery of ITD with precise pulse timing. The circles show the ITD thresholds of CI patients who lost their hearing either in adulthood (green circles), childhood (blue circles), or prelingually (red circles). For comparison, the dotted purple line shows the approximate ITD threshold one would expect to see in normally hearing humans [9], [14], [15], [16].
Figure 1: Comparison of ITD behavioral thresholds for interaural time differences (ITD) of 34 human CI patients (circles) with varying degrees of hearing experience versus 16 neonatally deafened CI rats (diamonds). The bilateral CI patients lost their hearing either in adulthood (green circles), in childhood (blue circles), or prelingually (red circles). The testing was performed using research processors that enable the presentation of informative pulse ITD. The black diamonds show the thresholds of 16 neonatally deafened bilateral CI rats. From the start of stimulation, all animals received informative ITD information on pulse timing and the envelope. Human data from [13]. Rat data from [25]. Figure modified from [48].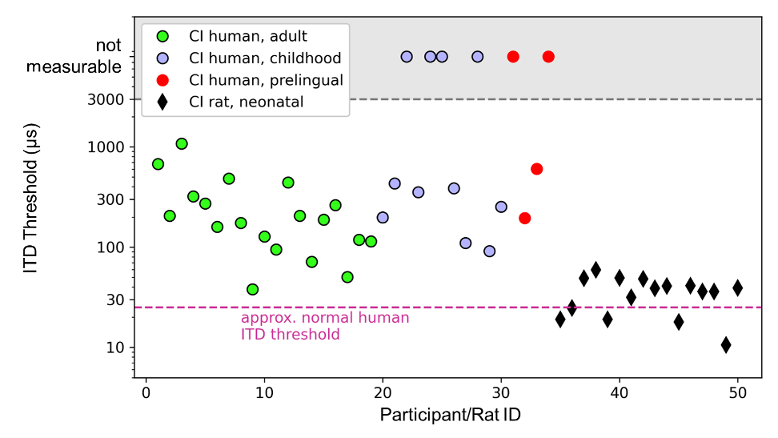
It is immediately apparent that only a tiny minority of human CI patients came close to matching the performance of normally hearing people. It should be noted that the data is plotted against a logarithmic y-axis in order to visualize the wide distribution of the often poor thresholds of many bilateral CI patients, and not because spatial hearing scales logarithmically with ITD in any way. The severity of the impairment of ITD sensitivity is therefore greater than it may appear visually. For source locations close to the midline, ITD scales approximately linearly with the source angle. The adult bilateral CI patients shown in Figure 1 [Fig. 1] have a mean ITD threshold of approximately 270 µs. This means that the spatial resolution of this binaural cue is on average approximately ten times worse than in normally hearing participants. Figure 1 [Fig. 1] also shows that CI patients who became deaf early in life, either as children (blue circles) or as babies (red circles), have an increased risk of poorer or even absent ITD sensitivity. In the study by Litovsky et al. [13], 4 out of 11 patients who became deaf in childhood, and 2 out of 4 patients who became prelingually, had no detectable ITD sensitivity. A later study by Ehlers et al. [17] confirmed that prelingually deaf CI patients exhibit particularly poor ITD sensitivity, and reported that 7 out of 10 patients had ITD thresholds >7,000 µs, and thus not measurable. In contrast, none of the 17 adult bilateral CI patients (Figure 1 [Fig. 1], green circles) had ITD thresholds >7,000 µs, even if their ITD thresholds were on average ten times worse than expected in normally hearing individuals.
The observation that early deaf CI patients often show poorer ITD sensitivity has led to the at first brush perfectly reasonable suggestion that early deafness may lead to a failure of the binaural timing circuitry of the auditory brainstem. Referring to the “critical period hypothesis”, a lack of auditory input during the early development of the auditory system was blamed for this [13], [17], [18]. However, while the human data reproduced in Figure 1 [Fig. 1] are indeed strongly suggestive of experience dependent plasticity playing a role, it clearly cannot be the only factor behind the poor ITD sensitivity outcomes in CI patients, given that adult deafened cohorts also exhibit greatly elevated ITD thresholds on average [13], [19], [20], [21], [22], [23].
Unfortunately, our ability to investigate all clinically relevant factors in human patient volunteers is very limited. For example, most or even all CI electrode channels of clinical CI processors only present accurate and thus informative pulse ITD under experimental conditions. In normal everyday use, however, the pulse ITD of neuroprosthetic signals are essentially random numbers, which are not only uninformative but also potentially highly misleading. The problem is that an auditory system preprogrammed by evolution expects to receive useful ITD information and to combine it with other cues to optimize spatial hearing. It is therefore possible that the auditory pathway may react to a sustained “diet” of inappropriate pulse timing by becoming desensitized to what would normally be a powerful temporal cue. Furthermore, this desensitization could be particularly pronounced in early deaf CI patients who had little or no experience with beneficial ITDs. However, investigating whether this is indeed the case is very difficult to do in a human patient population who have a clear clinical need to keep using the currently approved processing strategies in order to benefit, for example, from the valuable speech recognition cues that these devices do provide. To fully reap these benefits, patients typically have to invest many months of practice, as they learn to make the most of the specific, and quite unnatural, input provided by their particular devices. In doing so, learning to ignore uninformative patterns is likely just as important as learning to recognize and evaluate informative patterns. However, research into this possibility is fraught with serious technical and ethical limitations, given the risks of interfering in the patients’ journey towards making the most of the currently available standard of care. It is therefore difficult to avoid the data from human patients being affected by the effects of current clinical practice.
Improving ITD sensitivity: What the CI animal model teaches us
To overcome the limitations of human studies, we have developed a behavioral animal model that allows us to investigate ITD sensitivity to pulse timing during CI stimulation while fully controlling our animals’ acoustic or electric hearing experience. After demonstrating that normally hearing rats can learn ITD lateralization tasks easily and quickly and are able to distinguish minimal ITD of ~50 µs [24], we trained neonatally deafened (ND) rats on ITD lateralization who were fitted with bilateral CIs (biCI) in young adulthood [25], [26]. The results were remarkable. The black diamonds shown in Figure 1 [Fig. 1] demonstrate the behavioral ITD thresholds of 16 ND biCI rats tested with 300 pps pulse trains [25]. The mean ITD discrimination threshold for these animals is only 35 µs, and is not significantly different from those of normally hearing rats (sign rank test compared to comparable data from [24], p=0.16), while the mean ITD threshold of adult biCI patients is around ten times worde than in normally hearing humans (Figure 1 [Fig. 1]). None of our biCI animals had an elevated ITD threshold, even though they received no auditory input throughout their development until sexual maturity.
Our animal data are both surprising and highly encouraging, as they show that essentially normal ITD discrimination is at least in principle achievable through biCIs, even after prolonged hearing impairment throughout early life. This may seem surprising, especially in light of previous electrophysiological studies on congenitally deaf cats and neonatally deafened rabbits [27], [28], [29], [30], [31], [32], [33], which reported comparatively poor ITD sensitivity in the auditory midbrain or cortex of these early deaf animals. However, a careful look at these studies shows that the authors scanned ITD in very large steps and tested very few ITD values within the physiological range of the animals, which limits the usefulness of these data sets for predicting an animal’s likely ability to distinguish ITDs of ten microseconds. We therefore recorded ITD tuning curves of inferior colliculus (IC) neurons in ND biCI rats, focussing on the animals’ physiological range, and sampling ITDs in 20 µs steps. These electrophysiological measurements were performed under anesthesia, within hours after implantation, so that there was essentially no opportunity for experience dependent plasticity to shape the observed neural tuning curve. The observed responses are therefore indicative of the “raw”, hardwired state of an auditory midbrain that has grown up without either acoustic or prosthetic auditory stimulation. Even in this “raw”, inexperienced and developmentally deprived state, 85% of the recorded multiunits (groups of neurons) showed at least some sensitivity to ITD in the range of ±160 µs, and many multiunits modulated their firing rates significantly in response to ITD changes of only a few tens of microseconds, as shown in the example in Figure 2 [Fig. 2]. This figure shows a multiunit whose firing rate halves when the ITD changes from 20 µs to 60 µs (contralateral ear leading). These data thus demonstrate that the auditory midbrain of ND animals is capable of encoding abundant amounts of information about pulse timing ITDs with a resolution of tens of microseconds. This also explains why it is not difficult to train ND CI animals to lateralize small pulse ITDs.
Figure 2: Responses of a group of neurons (multiunit) in the IC to binaural electrical pulses with different ITDs. The data were measured in a neonatally deafened rat that was implanted with biCIs in young adulthood. Negative ITDs denote stimuli for which the ear contralateral to the recording site is leading. Left: Raster diagram, each blue dot shows an action potential, each row of dots shows the responses during a single trial, green and white bands group the responses to different ITDs as shown on the left. Right: Neuronal tuning curve for the same stimuli as on the left. The error bars show the SEM value of the response amplitude at 30 presentations of each ITD stimulus. Based on Fig. 2 from [49].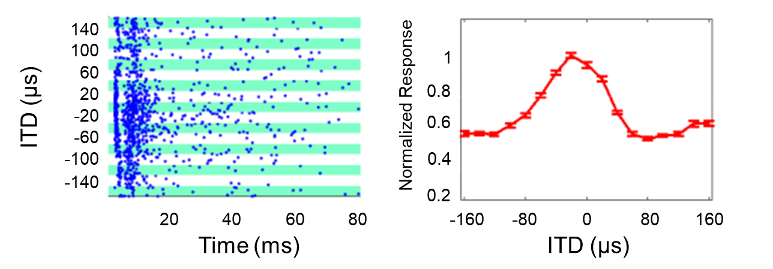
Our observation that biCI rats have no difficulty distinguishing very small ITDs has opened up new experimental avenues for investigating factors involved in the development of ITD sensitivity. We have already mentioned the possible influence of a “critical period” in development. This now appears to be less important, as the excellent ITD sensitivity shown in Figure 1 [Fig. 1] and Figure 2 [Fig. 2] originates from our biCI rats without early hearing experience during their development. Of course, humans develop much more slowly than rats, and early deaf human patients may undergo much longer periods of auditory deprivation in absolute terms than our ND rats did. We therefore cannot exclude the possibility that, for human CI patients, critical period effects may nevertheless play a role. Nevertheless, our animal data make the poor ITD sensitivity of human patients appear much less inevitable, irrespective of whether their hearing loss occurred very early or late in life.
To elucidate other factors that might contribute to the reduced ITD sensitivity in human CI patients, we have used our behavioral biCI rat model to answer two important questions: 1) Do ITDs really have to be delivered through pulse timing to be effective, or could pulse train envelopes be just as effective at delivering sub-millisecond temporal cues? 2) How does ITD sensitivity interact with other spatial cues such as interaural level differences (ILDs) in the prosthetically stimulated auditory system?
Studies on human patients investigating the relative effectiveness of pulse ITD compared to envelope ITD have already been conducted [19], [34], [35], [36]. Given the limitations faced by human studies described above, most of these studies could only examine patients who happen to have relatively good ITD sensitivity by the standards of biCI patients, whose auditory system had been adapted to clinical processors, and the experiments could only use stimuli with large ITD values of several hundred µs, at pulse rates of 50 to 400 pps, which are too low to be useful in clinical practice. With our ND biCI rat model, however, we were able to investigate the relative effectiveness of pulse timing and envelope ITDs in an auditory pathway that has not been conditioned by months of clinical processor use, using ITD values that are indicative of good binaural sensitivity (±80 µs), and at high pulse rates (900 and 4,500 pps). We showed that ND biCI rats were many times more sensitive to pulse timing ITDs than to envelope ITDs, regardless of the envelope shapes and pulse rates tested [37].
An obvious question is how pulse timing can favor ITD discrimination even at such high pulse rates, especially since one would expect that individual pulses cannot be resolved at rates above 4,000 pps. One possible answer to this question is that at high pulse rates, the auditory brainstem generates an onset response when the first pulse in the stimulus sequence is large enough to elicit a neural response in the auditory nerve. The results of animal experiments published by Hancock et al. [38] support this idea. In that case, the onset responses that the brainstem uses to time envelope features are still “locked to the temporal grids” set up by the pulse timing generators in each CI processor. Pulse timing then strongly determines temporal processing even at pulse rates so high that the auditory brainstem is no longer able to represent each pulse in a train individually. In any event, our experimental data clearly show that the timing of individual pulses matters a great deal, and that it cannot be substituted for with precise envelope timing. The decision by CI companies to make pulse timing in most or all electrode channels completely independent of the temporal characteristics of the incoming sound is therefore very likely to have consequences for CI patients. Recent modeling work by Josh H. McDermott’s group supports our conclusion. By simulating possible causes for the poorer hearing performance of CI patients in models of CI-mediated hearing, they provide important evidence that the usual clinical provision of temporal information exclusively on the envelope of the electrical stimulation is insufficient [39].
A bilateral CI patient with clinical processors currently receives essentially random pulse ITDs generated by the relative phase delay between the two pulse generators, which stimulate the left and right ears independently of each other. Assuming that the CI patient’s processors operate at pulse rates of approximately 1,000 pps, the respective channels in each ear receive approximately one pulse every millisecond independently of each other, and the pulse ITDs are therefore random numbers between zero (pulses are randomly synchronized) and 500 µs (pulse falls exactly in the middle between two pulses in the other ear). The phase relationship between the two processors also shifts in an unpredictable manner that is independent of the incoming sounds. The question is, what would we expect from a newly implanted, still inexperienced auditory system that has to process such uninformative, random pulse ITDs? In normal spatial hearing, ITD cues are unconsciously combined with ILDs and other cues to form an integrated perception of the source location. When ITD and ILD cues differ, the ear normally tries to find a compromise for localisation. For example, a sound that is slightly louder in the left ear but slightly earlier in the right ear may be perceived as lying on the midline. This is a phenomenon known as time-intensity trading. The relative strength of ITD and ILD cues in shaping overall spatial perception is called the time-intensity trading ratio (TITR) and is quantified in µs/dB [40], [41], [42], [43]. To understand the likely impact of randomized pulse timing ITDs delivered by clinical devices, it would be important to know TITRs for the CI stimulated auditory system in its native state. The more strongly TITRs are weighted in favor of ITDs (i.e., the smaller the TITRs), the greater the potential for random pulse timing ITDs to override useful ILD information and thus mislead CI patients about spatial height.
We recently completed experiments to investigate the interactions between ITD and ILD cues in ND biCI rats. Figure 3 [Fig. 3] shows a subset of these data [44]. The left panel of Figure 3 [Fig. 3] shows the waveforms of two stimuli used in the behavioral experiments. The biCI rats were trained and tested to localize stimuli in which the ILDs and pulse timing ITDs were varied independently. Positive ILD and ITD values favor the right ear and negative values favor the left ear. For clarity, we consider only two particularly informative stimuli from a larger set of test stimuli: a stimulus with –4 dB ILD (left ear louder) and 0 ITD (Figure 3 [Fig. 3], top left) and a stimulus with –4 dB ILD (left ear louder) and +80 µs pulse ITD (right ear leading in time) (Figure 3 [Fig. 3], bottom left). The right panel shows the percentage of cases in which the four ND biCI rats lateralized these stimuli to the right side. Given the clear ILD favoring the left side, all four animals lateralized the –4 dB/0 µs stimulus to the left in the vast majority (approximately 70%) of trials, as expected. However, in all four animals, we also see a statistically significant shift in perception to the right when the –4 dB ILD was paired with a +80 µs ITD. Three out of four animals now heard the stimulus predominantly on the right. Thus, a relatively small and contradictory ITD of only +80 µs was sufficient to extinguish a relatively strong ILD indication of –4 dB. In an early deafened and therefore inexperienced auditory pathway without prolonged conditioning with clinical processors, the TITR appears to be in the range of 20 µs/dB or smaller.
Figure 3: Behavioral data from four neonatally deafened rats with bilateral CIs trained in lateralization of binaural CI stimulus pulse trains of varying ITDs or ILDs. Left: Waveforms of the binaural pulse train stimuli considered in this example: the left ear pulses are 4 dB larger in amplitude than the right ear pulses, and they are delivered either synchronously (0 µs ITD, top), or very slightly earlier in the right ear (+80 µs ITD, bottom). Right: Percentage of trials on which each rat responded on the right as a function of the stimulus parameters shown on the x-axis. 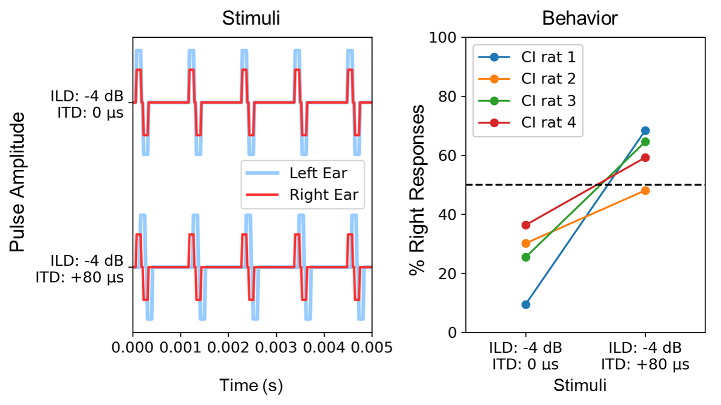
Assuming that the mechanisms for extracting and combining binaural cues are essentially similar across mammalian species, what would a TITR of around 20 µs/dB imply for a prelingually deaf patient who has just been fitted with a pair of clinical processors? Given the observations described above, we would not only expect this patient’s auditory system to be very sensitive to small pulse ITDs initially, but we would also expect this ITD sensitivity to be heavily weighted when ITD and ILD cues are combined. By simply extrapolating a TITR of ~20 µs/dB, one would predict that random pulse ITDs of, for example, 500 µs could interfere with informative ILDs of up to 25 dB. To put this number into perspective, we must take into account that in electrical CI hearing, the entire dynamic range of encodable stimulus intensities is significantly reduced with about 10 to 20 dB compared to acoustic hearing [45]. In that scenario, random pulse timing ITDs would be able to confound even the largest ILDs that clinical devices can possibly deliver. Admittedly, the linear extrapolation made here over such a large range of binaural cues may not be entirely valid, but the data in Figure 3 [Fig. 3] nevertheless clearly show that even a small “wrong” pulse timing ITD has the potential to mask a “correct” ILD. At present, it seems reasonable to assume that this situation also applies to human biCI patients whose auditory system is constantly bombarded with uninformative pulse timing ITDs from their clinical processors. We therefore assume that these patients must somehow “learn” to become insensitive to misleading pulse timing ITD if they are to be able to derive any benefits from the usually informative ILDs delivered by their devices. The poorer ITD sensitivity of CI users is therefore probably the result of an adaptation process in which the normally excellent temporal sensitivity of the binaural system is suppressed because the information it collects is only disruptive under clinical stimulation. The poorer ITD sensitivity of early deaf patients, as described in Figure 1 [Fig. 1], may be due to the fact that these patients had particularly little experience of useful ITD input, and were fitted with implants at a relatively early age, when the auditory pathway may still be more plastic, and learned to suppress the unhelpful ITD input. This process appears maladaptive in that it reduces the ITD sensitivity of the auditory system, which should actually be well developed but in this context only confuses the system. The hypothesis that prolonged exposure to uniformative pulse timing ITDs may lead to a decline in ITD sensitivity is in principle testable experimentally in our behavioral rat model, and experiments aiming to do that are currently underway in our laboratories.
For the sake of completeness, we would like to conclude by mentioning one exception of clinical processors by MED-EL, which since 2010 have been providing CI patients with pulse timing ITDs on four out of twelve stimulation channels via the FS4 and FS4p fine structure strategies by detecting the zero crossings of the acoustic signal and synchronizing to them [46]. This should, in principle, enable better ITD perception to be developed, at least in the lower-frequency channels, as the auditory system of these patients is supplied with informative pulse ITDs. A study by Fischer et al. [47] suggests that the advantage of FS4 fine-structure coding may be context-dependent. When FS4 CI patients were tested with low-frequency tones, they showed improved ITD discrimination in comparison to using a strategy without fine structure coding (HDCIS). However, this benefit was lost when the patients were tested with broadband noise in free field. One possible explanation for this is that with broadband signals, the majority of CI frequency channels (8 out of 12 stimulation electrodes) provide misleading pulse ITDs, masking the benefit of fine structure coding on channels 1 to 4. In natural hearing of broadband signals, ITD values are coordinated across all frequency channels. In summary, we believe it is important to provide biCI patients with as much more natural information as possible via electrical stimulation and, for example, to integrate the presentation of fine structure information into clinical processors. In our opinion, fine structure coding strategies represent an important step in the right direction. In the future, the provision of informative pulse ITDs across the entire cochlea could further improve spatial hearing in biCI patients.
Conclusion
Our data show that animal models are uniquely useful in the search for possible causes of limited hearing ability in CI patients. Using early deafened CI rats, we have shown that ITD sensitivity to CI stimuli can in principle be as good as in normally hearing individuals, even after substantial periods of auditory deprivation during early development. We also showed that good ITD sensitivity can be achieved even at relatively high clinical pulse rates (~900 pps). In experiments trading pulse timing and envelope timing cues off against each other, or trading pulse timing ITDs off against ILDs, we were able to confirm that, at sub-millisecond time scales, pulse timing matters greatly. It cannot be replaced by temporal envelope features, and the native sensitivity to pulse timing ITDs would be strong enough to disrupt the correct lateralization of ILD cues with CIs when the auditory system is exposed to random pulse timing. The data shown in Figure 3 [Fig. 3] thus suggest that the normally quite good ability of bilateral CI patients to use ILD cues may depend on their loss of sensitivity to ITDs. The decision by CI companies to make pulse timing completely independent of the temporal characteristics of the incoming sounds in most or all electrode channels is therefore likely to be a major cause of poor binaural hearing in bilateral CI patients.
Notes
Acknowledgments
We would like to thank Sarah Buchholz, Alexa N. Buck, Hendrike Budig, Cecilia Li, and Theresa Preyer for their support in the animal studies and data analysis. The work in this publication was supported by the German Academic Exchange Service (DAAD) with funds from the Federal Ministry of Education and Research (BMBF) and the “People” program (Marie Curie Actions) of the Seventh Framework Program (FP7/2007-2013) of the European Union, grant agreement no. 605728 (Postdoctoral Researchers International Mobility Experience), and by grants from the Hong Kong General Research Fund (11103524 & 11100219), the Hong Kong Health and Medical Research Fund (07181406 & 06172296), the Shenzhen Science Technology and Innovation Committee (JCYJ20180307124024360), the Martin Lee Centre for Innovations in Hearing Health at Macquarie University, the Research Commission of the Medical Faculty of the University Medical Center Freiburg, and the charity organization “Taube Kinder lernen hören e.V.”. Animal electrodes were kindly provided by MED-EL Medical Electronics, Innsbruck, Austria (research agreement PVFR2019/2).
Ethics
All procedures involving laboratory animals described here were performed in accordance with the approval of the Regierungspräsidiums Freiburg (No. 35-9185.81/G-17/124) and the Hong Kong Department of Health (No. 16-52 DH/HA & P/8/2/5). They were also approved by the Animal Research Ethics Sub-Committee of the City University of Hong Kong.
Competing interests
The authors declare that they have no competing interests.
References
[1] Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, Rabinowitz WM. Better speech recognition with cochlear implants. Nature. 1991 Jul 18;352(6332):236-8. DOI: 10.1038/352236a0[2] Zhu Z, Tang Q, Zeng FG, Guan T, Ye D. Cochlear-implant spatial selectivity with monopolar, bipolar and tripolar stimulation. Hear Res. 2012 Jan;283(1-2):45-58. DOI: 10.1016/j.heares.2011.11.005
[3] Quass GL, Kral A. Tripolar configuration and pulse shape in cochlear implants reduce channel interactions in the temporal domain. Hear Res. 2024 Mar 1;443:108953. DOI: 10.1016/j.heares.2024.108953
[4] Cariani PA, Delgutte B. Neural correlates of the pitch of complex tones. I. Pitch and pitch salience. J Neurophysiol. 1996 Sep;76(3):1698-716. DOI: 10.1152/jn.1996.76.3.1698
[5] Middlebrooks JC. Cochlear-implant high pulse rate and narrow electrode configuration impair transmission of temporal information to the auditory cortex. J Neurophysiol. 2008 Jul;100(1):92-107. DOI: 10.1152/jn.01114.2007
[6] Saddler MR, McDermott JH. Models optimized for real-world tasks reveal the task-dependent necessity of precise temporal coding in hearing. Nat Commun. 2024 Dec 4;15(1):10590. DOI: 10.1038/s41467-024-54700-5
[7] Borjigin A, Bharadwaj HM. Individual differences elucidate the perceptual benefits associated with robust temporal fine-structure processing. Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2317152121. DOI: 10.1073/pnas.2317152121
[8] Wohlbauer DM, Dillier N. A Hundred Ways to Encode Sound Signals for Cochlear Implants. Annu Rev Biomed Eng. 2025 May;27(1):335-69. DOI: 10.1146/annurev-bioeng-102623-121249
[9] Klumpp R, Eady H. Some measurements of interaural time difference thresholds. J Acoust Soc Am. 1956;28:859-60.
[10] Brown CH, May BJ. Comparative mammalian sound localization. In: Popper AN, Fay RR, editors. Sound Source Localization. New York: Springer; 2005. p. 124-78.
[11] Klump GM. How Does the Hearing System Perform Auditory Scene Analysis? In: van Hemmen JL, Sejnowski TJ, editors. 23 Problems in Systems Neuroscience. Oxford: Oxford University Press; 2006. p. 303-21.
[12] Ihlefeld A, Shinn-Cunningham B. Disentangling the effects of spatial cues on selection and formation of auditory objects. J Acoust Soc Am. 2008 Oct;124(4):2224-35. DOI: 10.1121/1.2973185
[13] Litovsky RY, Goupell MJ, Godar S, Grieco-Calub T, Jones GL, Garadat SN, Agrawal S, Kan A, Todd A, Hess C, Misurelli S. Studies on bilateral cochlear implants at the University of Wisconsin's Binaural Hearing and Speech Laboratory. J Am Acad Audiol. 2012 Jun;23(6):476-94.
[14] Zwislocki J, Feldman RS. Just Noticeable Dichotic Phase Difference. J Acoust Soc Am. 1956;28:152-3. DOI: 10.1121/1.1918072
[15] Brughera A, Dunai L, Hartmann WM. Human interaural time difference thresholds for sine tones: the high-frequency limit. J Acoust Soc Am. 2013 May;133(5):2839-55. DOI: 10.1121/1.4795778
[16] Thavam S, Dietz M. Smallest perceivable interaural time differences. J Acoust Soc Am. 2019 Jan;145(1):458. DOI: 10.1121/1.5087566
[17] Ehlers E, Goupell MJ, Zheng Y, Godar SP, Litovsky RY. Binaural sensitivity in children who use bilateral cochlear implants. J Acoust Soc Am. 2017 Jun;141(6):4264. DOI: 10.1121/1.4983824
[18] Kral A. Auditory critical periods: a review from system's perspective. Neuroscience. 2013 Sep 5;247:117-33. DOI: 10.1016/j.neuroscience.2013.05.021
[19] Majdak P, Laback B, Baumgartner WD. Effects of interaural time differences in fine structure and envelope on lateral discrimination in electric hearing. J Acoust Soc Am. 2006 Oct;120(4):2190-201. DOI: 10.1121/1.2258390
[20] Laback B, Majdak P, Baumgartner WD. Lateralization discrimination of interaural time delays in four-pulse sequences in electric and acoustic hearing. J Acoust Soc Am. 2007 Apr;121(4):2182-91. DOI: 10.1121/1.2642280
[21] Laback B, Egger K, Majdak P. Perception and coding of interaural time differences with bilateral cochlear implants. Hear Res. 2015 Apr;322:138-50. DOI: 10.1016/j.heares.2014.10.004
[22] Thakkar T, Anderson SR, Kan A, Litovsky RY. Evaluating the Impact of Age, Acoustic Exposure, and Electrical Stimulation on Binaural Sensitivity in Adult Bilateral Cochlear Implant Patients. Brain Sci. 2020 Jun 26;10(6):406. DOI: 10.3390/brainsci10060406
[23] Cleary M, Bernstein JGW, Stakhovskaya OA, Noble J, Kolberg E, Jensen KK, Hoa M, Kim HJ, Goupell MJ. The Relationship Between Interaural Insertion-Depth Differences, Scalar Location, and Interaural Time-Difference Processing in Adult Bilateral Cochlear-Implant Listeners. Trends Hear. 2022 Jan-Dec;26:23312165221129165. DOI: 10.1177/23312165221129165
[24] Li K, Chan CHK, Rajendran VG, Meng Q, Rosskothen-Kuhl N, Schnupp JWH. Microsecond sensitivity to envelope interaural time differences in rats. J Acoust Soc Am. 2019 May;145(5):EL341. DOI: 10.1121/1.5099164
[25] Buck AN, Buchholz S, Schnupp JW, Rosskothen-Kuhl N. Interaural time difference sensitivity under binaural cochlear implant stimulation persists at high pulse rates up to 900 pps. Sci Rep. 2023 Mar 7;13(1):3785. DOI: 10.1038/s41598-023-30569-0
[26] Rosskothen-Kuhl N, Buck AN, Li K, Schnupp JW. Microsecond interaural time difference discrimination restored by cochlear implants after neonatal deafness. Elife. 2021 Jan 11;10:e59300. DOI: 10.7554/eLife.59300
[27] Tillein J, Hubka P, Syed E, Hartmann R, Engel AK, Kral A. Cortical representation of interaural time difference in congenital deafness. Cereb Cortex. 2010 Feb;20(2):492-506. DOI: 10.1093/cercor/bhp222
[28] Tillein J, Hubka P, Kral A. Sensitivity to interaural time differences with binaural implants: is it in the brain? Cochlear Implants Int. 2011 May;12 Suppl 1:S44-50. DOI: 10.1179/146701011x13001035753344
[29] Hancock KE, Chung Y, Delgutte B. Neural ITD coding with bilateral cochlear implants: effect of binaurally coherent jitter. J Neurophysiol. 2012 Aug 1;108(3):714-28. DOI: 10.1152/jn.00269.2012
[30] Hancock KE, Chung Y, Delgutte B. Congenital and prolonged adult-onset deafness cause distinct degradations in neural ITD coding with bilateral cochlear implants. J Assoc Res Otolaryngol. 2013 Jun;14(3):393-411. DOI: 10.1007/s10162-013-0380-5
[31] Chung Y, Hancock KE, Delgutte B. Neural Coding of Interaural Time Differences with Bilateral Cochlear Implants in Unanesthetized Rabbits. J Neurosci. 2016 May 18;36(20):5520-31. DOI: 10.1523/jneurosci.3795-15.2016
[32] Tillein J, Hubka P, Kral A. Monaural Congenital Deafness Affects Aural Dominance and Degrades Binaural Processing. Cereb Cortex. 2016 Apr;26(4):1762-77. DOI: 10.1093/cercor/bhv351
[33] Chung Y, Buechel BD, Sunwoo W, Wagner JD, Delgutte B. Neural ITD Sensitivity and Temporal Coding with Cochlear Implants in an Animal Model of Early-Onset Deafness. J Assoc Res Otolaryngol. 2019 Feb;20(1):37-56. DOI: 10.1007/s10162-018-00708-w
[34] van Hoesel RJ, Tyler RS. Speech perception, localization, and lateralization with bilateral cochlear implants. J Acoust Soc Am. 2003 Mar;113(3):1617-30. DOI: 10.1121/1.1539520
[35] Laback B, Pok SM, Baumgartner WD, Deutsch WA, Schmid K. Sensitivity to interaural level and envelope time differences of two bilateral cochlear implant listeners using clinical sound processors. Ear Hear. 2004 Oct;25(5):488-500. DOI: 10.1097/01.aud.0000145124.85517.e8
[36] Noel VA, Eddington DK. Sensitivity of bilateral cochlear implant users to fine-structure and envelope interaural time differences. J Acoust Soc Am. 2013 Apr;133(4):2314-28. DOI: 10.1121/1.4794372
[37] Schnupp JWH, Buchholz S, Buck AN, Budig H, Khurana L, Rosskothen-Kuhl N. Pulse timing dominates binaural hearing with cochlear implants. Proc Natl Acad Sci U S A. 2025 Apr 22;122(16):e2416697122. DOI: 10.1073/pnas.2416697122
[38] Hancock KE, Chung Y, McKinney MF, Delgutte B. Temporal Envelope Coding by Inferior Colliculus Neurons with Cochlear Implant Stimulation. J Assoc Res Otolaryngol. 2017 Dec;18(6):771-88. DOI: 10.1007/s10162-017-0638-4
[39] Banerjee A, Saddler MR, McDermott JH. Understanding Cochlear Implants Using Machine Learning. In: Proceedings of the 47th MidWinter Meeting of the Association for Research in Otolaryngology, Anaheim, USA. 2024.
[40] George Moushegian LAJ. Role of Interaural Time and Intensity Differences in the Lateralization of Low-Frequency Tones. J Acoust Soc Am. 1959;31(11):1441-5.
[41] Durlach N, Colburn H. Binaural phenomena. In: Carterette E, Friedman ME, editors. Handbook of Perception. 4th ed. New York: Academic Press; 1978. p. 365-465.
[42] Trahiotis C, Kappauf WE. Regression interpretation of differences in time-intensity trading ratios obtained in studies of laterality using the method of adjustment. J Acoust Soc Am. 1978 Oct;64(4):1041-7. DOI: 10.1121/1.382087
[43] Joris PX, Michelet P, Franken TP, McLaughlin M. Variations on a Dexterous theme: peripheral time-intensity trading. Hear Res. 2008 Apr;238(1-2):49-57. DOI: 10.1016/j.heares.2007.11.011
[44] Buchholz S, Schnupp JWH, Arndt S, Roßkothen-Kuhl N. Interaural level difference sensitivity in neonatally deafened rats fitted with bilateral cochlear implants [Preprint]. bioRxiv. 2024. DOI: 10.1101/2024.07.30.605756
[45] Zeng FG, Grant G, Niparko J, Galvin J, Shannon R, Opie J, Segel P. Speech dynamic range and its effect on cochlear implant performance. J Acoust Soc Am. 2002 Jan;111(1 Pt 1):377-86. DOI: 10.1121/1.1423926
[46] Dhanasingh A, Hochmair I. Signal processing & audio processors. Acta Otolaryngol. 2021 Mar;141(sup1):106-34. DOI: 10.1080/00016489.2021.1888504
[47] Fischer T, Schmid C, Kompis M, Mantokoudis G, Caversaccio M, Wimmer W. Effects of temporal fine structure preservation on spatial hearing in bilateral cochlear implant users. J Acoust Soc Am. 2021 Aug;150(2):673. DOI: 10.1121/10.0005732
[48] Carlyon RP, Deeks JM, Delgutte B, Chung Y, Vollmer M, Ohl FW, Kral A, Tillein J, Litovsky RY, Schnupp J, Rosskothen-Kuhl N, Goldsworthy RL. Limitations on Temporal Processing by Cochlear Implant Users: A Compilation of Viewpoints. Trends Hear. 2025 Jan-Dec;29:23312165251317006. DOI: 10.1177/23312165251317006
[49] Buck AN, Rosskothen-Kuhl N, Schnupp JW. Sensitivity to interaural time differences in the inferior colliculus of cochlear implanted rats with or without hearing experience. Hear Res. 2021 Sep 1;408:108305. DOI: 10.1016/j.heares.2021.108305




