[Factors that influence the outcome of cochlear implant fitting: Is there a relationship between electrode insertion angle/frequency mismatch and monosyllabic intelligibility?]
Emily Gooss 1Uwe Baumann 1
1 Clinic for ENT Medicine, Goethe University Frankfurt, University Hospital Frankfurt a.M., Germany
Abstract
Objective: The objective of this study is to examine the efficacy of cochlear implants (CIs) in restoring hearing in individuals with hearing impairment. Cochlear implants (CI) are medical devices that are used to restore the hearing of patients who are deaf or hard of hearing. Nevertheless, achieving optimal hearing outcomes remains challenging. The existing literature has addressed the potential factors that may influence these outcomes; however, these factors have not been fully elucidated. The present study aims to address this knowledge gap by analyzing the effects of Angular Insertion Depth (AID) and Frequency Mismatch (FMM) on Speech Understanding (SU) in a large cohort of patients with five different electrode arrays.
Methodology: N=101 implant cases were included in the study. Electrode insertions of the following implants were examined: N=43 Synchrony implants (MED-EL, Innsbruck, Austria) with FLEX26 and FLEX28 electrodes, and N=58 Nucleus implants (COCHLEAR, Macquarie, Australia) with PRECURVED, STRAIGHT and CONTOUR SLIM electrodes. Postoperative CT data sets were analyzed using the Otoplan V3.0 analysis program (CASCINATION, Bern, Switzerland) to determine the electrode positions. The tonotopic frequency allocation and the FMM were then evaluated. Postoperative speech comprehension was determined for all subjects using the Freiburg monosyllable test in quiet (FMT) after an average of 12.2±5.9 months.
Results: The mean CDL (cochlear duct length) was 35.8±3.1 mm (N=101). The AID values showed the following results: PRECURVED 329.4±39.7° (N=34), STRAIGHT 344.2±34.4° (N=9), CONTOUR SLIM 394.3±37.3° (N=10), FLEX26 496.3±45.0° (N=10) and FLEX28 554.8±49.3° (N=33). The largest FMM was found at the most apical electrode. Independent of the manufacturer, the cohort with AID 400–450° (N=9) achieved the best speech discrimination with 80%.
Conclusion: The use of Otoplan V3.0 in clinical practice for the determination of CDL and AID was found to be practicable. No significant correlation was found between FMM and FMT, nor between AID and FMT. The results suggest that FMM may not be as crucial for speech comprehension of monosyllabic test words as previously assumed.
Keywords
cochlear implant, frequency-to-place mismatch, speech perception, angular insertion depth, tonotopy
1 Introduction
Multi-channel cochlear implants (CI) have been commercially available since the 1980s and enable hearing to be restored in deaf and hard of hearing patients [34]. Current CI systems consist of an internal stimulator with an electrode array and an external audio processor, which inductively transmits sound waves converted into electrical signals to a receiver coil. The stimulator uses the electrode array inserted into the cochlea to generate electrical stimuli for direct stimulation of the auditory nerve, resulting in an auditory perception in implanted patients [9]. Notwithstanding the extensive success of cochlear implant rehabilitation, satisfactory hearing results are not always achievable, especially in unfavourable acoustic conditions, where a significant proportion of cochlear implant patients do not achieve satisfactory speech understanding (SU) [23]. Despite long-term adaptation to cochlear implant hearing, music appraisal is frequently reported as inadequate [4]. An essential component of contemporary research in the domain of CI pertains to the relationship between speech intelligibility, subjective hearing quality, and the insertion depth or the insertion angle of the CI electrode array, or the apical section of the electrode array. Another research question investigates whether a smaller frequency mismatch/frequency shift (FMM) between the tonotopy of the basilar membrane and the “pseudo-tonotopy” specified by the electrode position and fitting setting (frequency assignment) leads to better SU. The basilar membrane as the central structure in the cochlea separates the cochlear duct (scala media) from the scala tympani, and serves as the basis for the organ of Corti (OC), which contains the sensory hair cells. The membrane shows a gradual change in width along its length: it is narrow at the base of the cochlea and becomes wider towards the apex. This anatomical variation correlates with the function of the membrane, as low-pitched sounds are represented in the wider apical region, while high-pitched sounds are mapped to the narrower basal part of the cochlea. Using a formula developed by Greenwood in 1961 [14], the spatial frequency can be described as a function of the distance to the round window. The frequency range is distributed logarithmically along the OC. The FMM, which results from the difference between the assigned center frequency of a specific electrode frequency band and the actual spatial frequency, may be a critical determinant of hearing outcomes in CI patients. The model developed by Stakhovskaya et al. [31] is often used to describe the assignment of the electrode position to the stimulated frequency range more precisely. This involves differentiating between stimulation near the spiral ganglion (SG) and stimulation near the hair cells of the organ of Corti. Recent studies have shown that the length of the human cochlear duct (cochlear duct length, CDL) [5], [11], [12], the insertion angle of the most apical electrode (angular insertion depth, AID) [5], [16], [19] and both the shape and length of the electrode array [19] can affect the FMM. Canfarotta et al. [5] examined two electrode arrays of different lengths from the manufacturer MED-EL (Innsbruck, Austria) and determined an average AID of 428° for 24 mm long electrodes (FLEX24) and 558° for 28 mm long electrodes (FLEX28). The SU was measured after 1, 3 and 6 months. After excluding data that deviated significantly from the mean (“outliers”), no significant correlation between FMM and SU was found. Mertens et al. [25] determined an FMM of 17 semitones in the mean value at higher frequencies for the most apical electrode of the FLEX28 carrier. As with Canfarotta et al. [5], no correlation between FMM and SU could be determined. Landsberger et al. [19] found that longer electrode arrays (FLEX28) showed a lower FMM in the stimulation range for frequencies below 650 Hz than shorter arrays (HiFocus 1J and Contour Advance), while there were no significant differences in FMM between the electrode arrays for frequencies above 650 Hz. Dutrieux et al. [11] studied 99 patients supported with 106 FLEX28 electrode arrays and described significant correlations between CDL, AID and FMM. The largest FMM was found at the most apical electrode. In a study by Chakravorti et al. [7], the SU was investigated in MED-EL and COCHLEAR electrode arrays, taking into account influencing factors such as AID, gender and CI usage time. However, the FMM was not included as a possible influencing factor and contemporary research does not demonstrate a definitive association between FMM and SU. The significance of the parameters CDL and AID on the outcome of CI rehabilitation does not appear to be fully clarified. In particular, there is a lack of comprehensive comparative studies that systematically examine the electrode arrays of different manufacturers, which represents a significant limitation in the current state of knowledge. In the present retrospective study, the parameters CDL, diameter, width and height of the cochlea, AID, frequency assignment, FMM and individual monosyllabic intelligibility after 12 months (FMT) were therefore recorded in a group of patients supported with five different types of electrodes (manufacturers: MED-EL and COCHLEAR) and examined for correlations. The test interval 12 months after the initial fitting of the CI processor was chosen because studies show that there is only a slight increase in SU at the test interval after 24 months. Thus, after 12 months, an extensive adaptation to the individual FMM can be assumed [20], [29], [30]. As part of the comparison of the electrode supports, Otoplan was also used for the first time for electrodes from the manufacturer COCHLEAR. The main objective of this research work is to determine a potential correlation between the AID, the associated FMM and the FMT. In addition, the aforementioned parameters are analyzed for intercorrelations to describe possible associations, with the goal of enhancing the quality of CI fitting.
2 Material and methods
2.1 Patient cohort
A query of the internal CI database of the University ENT Clinic Frankfurt revealed N=236 ears supported with CI in adulthood in the period from 2017 to 2021. By applying the inclusion criteria “German as mother tongue” and available post-operative CT image data, N=229 remained. Cases without FBE test results (N=6) and cases with auditory nerve deficiencies (N=2) were excluded. After the exclusion of non-evaluable CT data sets (N=8) and cases supported with electrical-acoustic or hybrid stimulation (N=4), N=190 CI-supplied ears remained. Due to time constraints, a sample of N=101 cases was selected. The selection was randomized by an employee of the audiology department of the University Hospital Frankfurt who was not involved in the study. The selected sample size was considered approximately sufficient to enable a representative analysis. This left N=101 CI-supported ears (N=55 female and N=46 male; average age 57±15 years). The cause of hearing loss remained unknown in N=67 cases. Known causes were idiopathic sudden deafness (N=13), followed by chronic progressive hearing loss (N=6) and congenital causes (N=5). With N=3 each, infections and meningitis are the causes. Otosclerosis, genetic causes, mucopolysaccharidosis type II and Meniere’s disease are represented in the cohort with N=1 each. The choice of electrodes was only made by the surgeon in the case of special anatomical or pathological conditions. In all other cases, this decision was made by the patient during a detailed consultation. The electrode type groups investigated were homogeneous in terms of age, gender and aetiology.
2.2 Implants and electrode arrays
A total of N=43 Synchrony implants with the electrode arrays FLEX26, FLEX28 (MED-EL, Innsbruck, Austria) and N=58 Nucleus implants with the electrode arrays PRECURVED (CI512, CI612), STRAIGHT (CI522, CI622) or CONTOUR SLIM (CI532, CI632) from the manufacturer COCHLEAR (Macquarie, Australia) were used (Table 1 [Tab. 1]). Deactivated electrode contacts and incompletely inserted electrode arrays are listed in Table 1 [Tab. 1] according to electrode type. Electrode arrays with complete insertion had deactivated electrodes as follows: 5x1, 6x2, 4x3, x4 and 1x6 deactivated electrodes. For incompletely inserted electrode arrays, the following variants were observed: 2x2, 2x3 and 1x6 deactivated electrodes. Cases with incompletely inserted electrode arrays (STRAIGHT N=4, PRECURVED N=1) were excluded from the evaluations of AID and FMM in order to avoid distortion of the results.
Table 1: Electrode arrays with frequencies, electrode length in mm, number of electrode arrays with incomplete insertion and number of electrode arrays with deactivated electrode contacts
2.3 CI processors and CI rehabilitation
The following speech processors were used by MED-EL users: SONNET, SONNET 2, SONNET EAS, RONDO 2, RONDO 3. For users of COCHLEAR implants, CP1000, CP950, CP920, CP910, CP810 and KANSO 2 speech processors were used. After CI fitting, all patients were prescribed systematic rehabilitation, either as part of a three- to five-week inpatient stay at a specialized rehabilitation facility or by completing 20 outpatient rehabilitation sessions, usually within 12 months. The patients received appointments for medical and audiological checks of the CI system after 3, 6 and 12 months and annually thereafter.
2.4 Analysis of the image data
The CT data sets were extracted from the PACS (Picture Archiving and Communication System, GE Healthcare, Frankfurt) of the University Hospital Frankfurt and then imported into the image data reconstruction program Otoplan V3.0 (CAScination AG, Bern, Switzerland). To determine the CDL, the sectional image data was aligned in all three spatial planes to define its boundaries and the position of the round window. The elliptic-circular approximation (ECA) method was used for the calculation (Attachment 1 [Att. 1], Appendix 1). The method used the following equation:
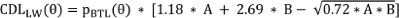
with:
CDL(LW)(θ): Location on the lateral wall (LW) of the cochlea as a function of θ
A: Diameter of the cochlea, measured linearly from the center of the round window to the outermost point of the opposite side wall (according to Escudé et al. [12])
B: Width of the cochlea, measured perpendicular to A, through the modiolus axis
pBTL: Constants defined for each angle θ along the cochlea
2.5 Determination of the insertion angle
In the first approach, the position of the electrodes was detected using Otoplan V3.0, which was then checked and corrected manually if necessary. To determine the AID of the individual electrodes, the cochlea had to be aligned as described in the previous section. In addition, a zero reference angle was determined, a reference line extending from the center of the round window to the modiolus, the center of the cochlea. The determination of the angular position of the electrodes was then read out from Otoplan using this reference line as a reference.
2.6 Determination of the electrode position and tonotopic assignment
The tonotopic assignment of the most apical electrode position (electrode 22 for nucleus implants, electrode 1 for synchrony implants) was performed for two reference planes within the cochlea: Plane of Corti’s organ (OC) and plane of the spiral ganglion (SG). The corresponding information on the tonotopic assignment was taken from the tabular listing in Otoplan.
2.7 Determining the frequency assignment
A standard frequency assignment was available for most patients in the cohort. The individual frequency assignment was determined for Synchrony implants by looking at the CI processor setting used at the time of the speech test using the manufacturer’s clinical software (MAESTRO 9.0, MED-EL, Innsbruck). The transmission frequency range extends from 70 to 8,500 Hz for the standard frequency assignment, which is distributed logarithmically over the 12 active electrodes [10]. The apical electrode then has a center frequency of fm=120 Hz. For MED-EL implants, the standard transmission frequency range extends from 170 to 7,200 Hz. The standard center frequency at the most apical electrode is 242.6 Hz. In some patients, a frequency allocation deviating from the standard was made. The center frequency of the most apical electrode was shifted to higher frequencies. This preferentially affected FLEX26 and STRAIGHT electrode arrays. Regardless of the implant type, extracochlear electrode contacts were deactivated and the frequency filters were redistributed accordingly to the remaining electrode contacts.
2.8 Determination of the frequency mismatch
The FMM is calculated from the difference between the center frequency of the frequency band assigned to an electrode as determined by the CI processor setting and the tonotopic assignment determined by the position of the electrode. This discrepancy occurs when the assignment of the frequency bands in the speech processor does not match the natural tonotopic organization of the cochlea.
2.9 Postoperative speech intelligibility
The results of the Freiburg monosyllabic test in quiet were determined for all subjects at a free-field presentation level of 65 dB SPL. Data from the control 12 months after initial fitting was mainly used for the evaluation. As this control date had to be postponed in individual cases (e.g. after delayed start of rehabilitation), the average time of this control was 12.2±5.9 months. All audiometric measurements were performed in sound-isolated rooms from IAC Acoustics (Winchester, UK) equipped with Equinox 2.0 clinical audiometers (Interacoustics, Middelfart, Denmark) and CD 220.3 loudspeakers (Canton Elektronik GmbH, Weilrod, Germany) for free-field presentation. Calibration was checked regularly by a calibration service in accordance with the manufacturer’s instructions. The patients used the usual everyday settings of their CI processors for the speech audiometry test.
2.10 Statistics
The statistical analysis was performed with SPSS Statistics 28.0 (IBM Corporation, Endicott, NY, U.S.A.). Descriptive statistics and boxplots were used to present the data. Results are presented as median ± standard deviation (SD). Spearman rank correlation was used for data not subject to normal distribution. Pairwise comparisons for independent samples were calculated using the Kruskal-Wallis test. A p-value of p<0.05 was considered statistically significant.
3 Results
3.1 Cochlear parameters
The ECA method resulted in a mean CDL of 35.8 mm (Md=36.0 mm, N=101, SD=3.1) with a range of 25.8 to 46.2 mm. The mean diameter of the cochlea was A=9.2 mm (SD=0.7), the width B=6.8 mm (SD=0.7) and the height H=3.8 mm (SD=0.5). There was no significant correlation between CDL and the number of deactivated electrodes (r=0.082, p=0.414).
3.2 Insertion angle (AID)
The list of AID for the most apical electrode, divided by electrode type, is shown in Table 2 [Tab. 2]. There was a significant correlation between CDL and AID of the most apical electrode for FLEX28 users (r=–0.396, p=0.023) and PRECURVED electrodes (r=–0.481, p=0.003). FLEX26 (r=–0.576, p=0.082), STRAIGHT (r=–0.418, p=0.156) and CONTOUR SLIM electrodes (r=–0.539, p=0.108) showed no significant correlation.
Table 2: List of insertion angles (in angular degrees, °) for the most apical electrode, subdivided by electrode type; incompletely inserted electrodes (N=5) excluded; standard deviation (SD)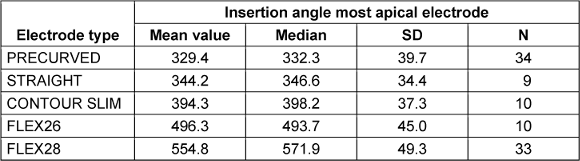
3.3 Tonotopic assignment
The tonotopic assignment depending on the electrode insertion angle was carried out both at the level of the spiral ganglion (SG) and at the level of the organ of Corti (OC) (Figure 1 [Fig. 1]).
Figure 1: Boxplot, tonotopic assignment (in Hz), determined from the electrode insertion angle of the most apical electrode for different electrode supports. SG-Freq (blue): Spiral ganglion plane, OC-Freq (red): Organ of Corti plane. N-values see Table 1, excluding incompletely inserted electrodes (N=5), (numerical values see Tab. 4 in Attachment 1, Appendix 2)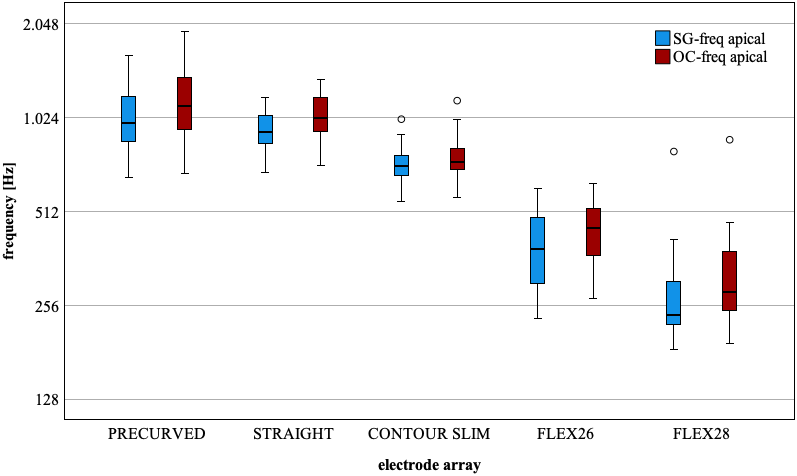
3.4 Frequency mismatch
The FMM determined in semitone steps is listed in Table 3 [Tab. 3] for the electrodes tested. The FMM is greater at the OC than at the SG for each electrode type.
Table 3: Frequency mismatch measured in semitone steps for the most apical electrode, differentiated by SG, OC and electrode type, FMM: frequency mismatch. Incompletely inserted electrodes (N=5) excluded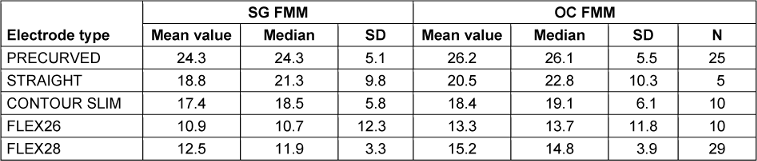
A Kruskal-Wallis test shows that the FMM in semitone steps (i.e. frequency ratio two to the power of one twelfth per semitone step) is influenced by the type of electrode (SG-FMM H=64.060, p<0.001, df=4; OC-FMM H=63.991, p<0.001 df=4). Subsequent post-hoc tests (Dunn-Bonferroni tests) show that the electrode arrays FLEX28 and STRAIGHT (H=29.470, SE=10.367, p=0.045), FLEX28 and PRECURVED (H=51.348, SE=6.787, p<0.001) and FLEX26 and PRECURVED electrodes (H=48.795, SE=9.951, p<0.001) differed significantly at the SG of the most apical electrode. The results of the analysis of variance are shown in Tab. 5 in Attachment 1 [Att. 1], Appendix 2. The remaining comparisons showed no significance.
3.5 Intelligibility of monosyllabic test words
There was no significant difference between the different electrode array types with regard to intelligibility of monosyllabic test words (H=6.609, p=0.158, df=4). In order to perform an analysis that was independent of electrode type, the AID of the most apical electrode was divided into 50° steps, and the results of the FMT were divided into the corresponding AID categories (Figure 2 [Fig. 2]).
Figure 2: Boxplot, overview of grouped insertion angles of the most apical electrode, manufacturer-independent, results of the Freiburger monosyllabic intelligibility test (%), here corresponding to the values from Tab. 6 in Attachment 1, Appendix 2, excluding incompletely inserted electrodes (N=5)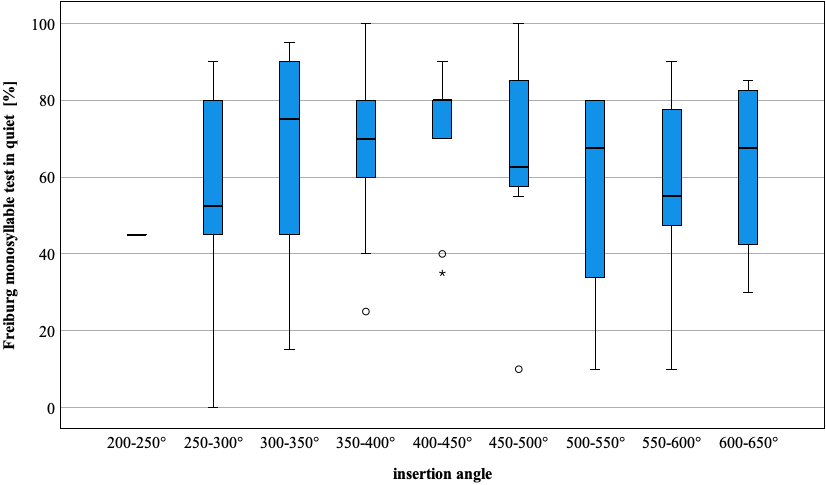
3.6 Correlations
The results of the Freiburger monosyllabic intelligibility test were correlated with the FMM (related to OC and SG level) of the most apical electrode. The results of the rank correlation according to Spearman were not significant (SG-FMM r=0.017, p=0.867; OC-FMM r=0.018, p=0.859). The correlation between the results of the FMT and the age of the patients with CI fitting (r=–0.022, p=0.826), as well as that between FMT and AID of the most apical electrode (r=–0.048, p=0.645), showed no significance. A comparison between patients with and without deactivated electrode contacts was not significant with regard to FMT (p=0.351, df=1). The aim of the present study was to investigate the influence of the AID of the electrode tip and the influence of the FMM with five different electrode arrays on the SU in a large group of patients. The results of the statistical analyses suggest that the FMM may not be as decisive for the SU of monosyllabic test words as previously assumed.
4 Discussion
4.1 Cochlear duct length (CDL)
The CDL parameter was determined using the Otoplan Version 3.0 system and resulted in an overall mean value of 35.8 mm (N=101, SD=3.1). The results obtained show good agreement with the results of a meta-analysis by Koch et al. [17], which determined a comparable value of 35.04 mm (29.7–38.9 mm range). In a study by Dutrieux et al. [11], only patients with a FLEX28 electrode array fitting were examined (N=106). The CDL was determined using OTOPLAN version 1.2 and the Alexiades formula [1]. A CDL of 34.5 mm was determined. The results of the study presented here show broad agreement with those from the literature. The analysis of CT data sets using the reconstruction algorithms implemented in the Otoplan software thus proved to be a suitable method for determining the CDL. In view of the significant variability of the CDL, the selection based on a previously determined individual CDL for the electrode array design appears to make sense in principle.
4.2 Does incomplete insertion affect speech intelligibility?
Basally located electrodes can be deactivated either due to incomplete insertion or as part of the postoperative fitting of the CI processor due to unpleasant hearing sensations. It is to be expected that a smaller cochlea size results in a higher probability of incomplete electrode insertion. Proportional evaluation revealed that patients with the shorter STRAIGHT electrode were more likely to have deactivated and incompletely inserted electrodes (Table 1 [Tab. 1]). This could be due to the design of this electrode array with the basal electrodes relatively close to the round window. Heutink et al. [15] investigated the effect of incompletely inserted electrodes on the SU. In 13 patients with incompletely inserted PRECURVED or STRAIGHT electrode arrays in the study, there were no statistically significant differences in SU compared to fully inserted electrodes. These observations could be confirmed with the data obtained in the present study. Incomplete CI implantation and deactivation of the electrodes had no significant effect on the SU in the cohort examined here and confirm the observations of Heutink et al. [15].
4.3 Insertion angle (AID)
The varying AIDs of the individual electrode types result from their different lengths and contour shapes. In addition, the individual anatomical features of the cochlea and the distribution of the electrodes along the electrode array must be taken into account. The PRECURVED electrodes (CI512, CI612) have a predetermined curvature. The AID of the most apical electrode reaches 332.3° (N=34, SD=39.7) and thus shows the lowest value in the group comparison. In a study by Peters et al. [27], an AID of 307.3° was determined for CI512 electrode carriers in a patient number of N=6. In a study by Canfarotta et al. [6] , N=7 PRECURVED electrodes resulted in an AID of 364°. The STRAIGHT electrode (CI522, CI622) resulted in an AID [22] of 346.6° (N= 9, SD=34.4) due to its proximity to the lateral wall. In a systematic literature review, Breitsprecher et al. [3] determined a slightly larger AID of 370° for STRAIGHT electrodes. The CONTOUR SLIM electrode is the shortest of the electrodes investigated, measuring 14 mm (see Table 1 [Tab. 1]). The design of this electrode support enables a large AID due to the close fit to the modiolus with a short length, resulting in a value of 398.2° (N=10, SD=37.3). These results are in good agreement with the average AID of 406° determined by McJunkin et al. [24] (implant model CI532). In a study by Ketterer et al. [16], the FLEX26 electrode showed an average AID of 517° (N=15), which was slightly higher than the value determined in this study (AID=493.7°, N=10, SD=45.0), but due to the large scatter of the data, it falls within the scope of the results. The FLEX28 electrode is the longest of the electrode supports examined here. The mean AID for this electrode was 571.9° (N=33, SD=49.3), slightly higher than the value reported by Canfarotta et al. [5] (558°). The statements made in earlier studies by Canfarotta et al. [5], [6], Ketterer et al. [16] and Venail et al. [32], according to which longer FLEX28 electrode arrays have a larger AID compared to FLEX26 electrodes, were confirmed by our study results. The observation made by Heutink et al. [15] that PRECURVED electrodes achieve greater insertion depth within the cochlea compared to STRAIGHT electrodes could not be confirmed in the present study. This could be due to the relatively small number of patients with STRAIGHT electrodes (N=9, PRECURVED electrodes N=34). The use of Otoplan Version 3.0 therefore also proved to be a suitable tool for precisely determining the AID parameter for electrodes from the manufacturer COCHLEAR.
4.4 Correlation between CDL and AID
The correlation between CDL and AID at the most apical electrode was significant for FLEX28 and PRECURVED electrodes. No significant correlation could be demonstrated for FLEX26, STRAIGHT and CONTOUR SLIM electrodes. Canfarotta et al. [5] investigated the relationship between CDL and AID in FLEX electrode arrays. A significant correlation was found with fully inserted electrode arrays (p<0.05). Dutrieux et al. [11] also found a significant correlation between CDL and AID in FLEX28 electrodes (N=106). The author found the strongest correlation at the most apical electrode (p<0.0001). The results of previous studies could therefore be confirmed with the data presented here, at least for FLEX electrode arrays. The negative sign of the correlation confirms that larger CDL values result in a lower AID, as expected. If a certain insertion depth—measured by the AID—is to be achieved, it is advisable to determine the individual CDL. However, according to Pietsch et al. [28], anatomically determined variations of the cochlea can occur both on the lateral and preferably on the modiolar wall, which counteract the achievement of an insertion angle corresponding to the preoperative planning. Breitsprecher et al. [2] also found deviations in the AID of 5% in preoperative planning using CT, which may require the use of a correction factor.
4.5 Frequency mismatch
In this study, an average FMM was determined in semitone steps for the electrode supports examined. The FMM decreases with a larger AID. The correlation analysis between AID and FMM showed a strongly negative, highly significant correlation (r=–0.905, p<0.001). This allows the conclusion that the FMM decreases with greater AID. The mean spatial frequency for the most apical FLEX28 electrode is about 12 semitones above the SG frequency. This deviation is about 11 semitones for the FLEX26 electrode, while the CONTUR SLIM electrode showed deviations of 18 semitones. The group of STRAIGHT electrodes showed an FMM of 21 semitones. This decreased by about 3 semitones when all cases were considered in the evaluation. The PRECURVED electrode showed the largest FMM of 24 semitones. Neumayer et al. [26] observed a similar apical FMM for FLEX28 electrodes, while Mertens et al. [25] even described an FMM of over 17 semitones. In agreement with the value determined in this study, Canfarotta et al. [5] reported a similar FMM for FLEX28 electrodes at the most apical electrode. Dutrieux et al. [11] also described a FMM of 10–16 semitones at the most apical electrode for FLEX28 electrodes, based on the Greenwood Map [13]. Landsberger et al. [19] determined a larger FMM for shorter electrode arrays (HiFocus 1J and Contour Advance) apically (below 650 Hz) than for longer electrode arrays (FLEX28), while there were no significant differences in the FMM between the electrode arrays further basally (above 650 Hz). This larger FMM for shorter electrode arrays is consistent with the results of the PRECURVED and CONTOUR SLIM electrodes in this study. The results of the FMM at the most apical electrodes confirm the results of previous studies and suggest that the length of the electrode array influences the FMM and, as expected, shorter electrodes lead to a higher FMM of the most apical electrode. Somewhat unexpectedly, the shorter FLEX26 electrode showed a smaller deviation in FMM compared to the FLEX28 electrode. This can be explained by an increase in the lowest transmission frequency in patients with residual low-frequency hearing. In 6/10 patients, a setting deviating from the default frequency map was applied for higher frequencies.
A significant difference in the FMM between the FLEX28 and CONTOUR SLIM electrodes could be demonstrated. After performing the Bonferroni correction for multiple comparisons, there is a trend towards significance (p=0.055, see Tab. 5 in Attachment 1 [Att. 1], Appendix 2). It should be noted that the patient population for the CONTOUR SLIM electrodes (N=10) is smaller than that for the FLEX28 electrodes (N=33).
4.6 Relationship between speech intelligibility and frequency mismatch
The rehabilitation outcome of the patients in the present study was determined using the results of the Freiburg monosyllabic test after 12 (±6) months. The available data showed no significant correlation between FMM and FMT. It is conceivable that the adaptation of the respective subject to the individual FMM after 12 months leads to the absence of a significant influence on the FMT. Canfarotta et al. [5], [6] investigated the influence of the FMM at 1,500 Hz on the CVC test result in FLEXSOFT, FLEX28 and FLEX24 CI arrays using electrical stimulation only (N=48). Lower FMM predicted better CNC results at one month (r=–0.367, p=0.010), three months (r=–0.334, p=0.021) and six months (r=–0.401, p=0.005). Videhult et al. [33] compared the SU achieved with CONTOUR SLIM and PRECURVED electrodes and found no significant differences in SU between the two groups 1 year postoperatively. In a systematic literature review by Breitsprecher et al. [3], no significant correlation between AID and speech perception was found in seven studies. In contrast, a significant correlation or a positive effect was found in fifteen studies. One study also showed a significant negative correlation. Chakravorti et al. [7] conducted studies on MED-EL models (N=50) and COCHLEAR models (N=120), where no correlation was found between the AID parameter and the SU (CNC Words and BKB-SIN). So far, mainly longer electrode arrays have been investigated with regard to the relationship between FMM and FMT. Only one study with shorter electrode arrays (Advanced Bionics) is available [18]. This showed the opposite effect, namely that a reduction in FMM leads to a deterioration in SU. In agreement with numerous published studies, the result of the SU after 12 months in the patient cohort examined here shows no statistically verified correlation with the FMM. This leads to the conclusion that the AID parameter cannot be regarded as a decisive criterion for the SU either.
4.7 Limitations of the study
It can be assumed that for the most apical electrode, the “SG” position tends to predominate for preformed electrode arrays and the “OC” position for straight electrodes. Due to the limited resolution of the CT scan (layer thickness 0.4–1 mm), it was not possible to determine the position in a defined manner, which is why both positions are listed. Due to the hardening artifact of the platinum electrodes in the imaging, the assessment of scale changes is only possible to a very limited extent and with great uncertainty and was therefore not performed. The low number of cases of the FLEX26 and CONTOUR SLIM electrode arrays could affect the validity of the results. The different distances between the individual electrodes in different electrode arrays harbor the potential for inaccuracies in the determination of the FMM by Otoplan. In SSD patients, an individual pitch comparison with the acoustically hearing opposite ear could be used to achieve a more accurate assignment of the filter center frequency. Other aspects possibly influenced by the electrode design such as sound quality or listening effort as well as the influence of individual cognitive abilities on SU with CI and SU in noise were not the subject of this study. Psychoacoustic effects were not considered.
5 Conclusion
The data collected in the present study largely confirmed the values for CDL and AID reported in the literature for the electrode arrays investigated here. A significant correlation between CDL and AID was found for FLEX28 and PRECURVED electrodes. In addition, it was confirmed that longer electrode arrays lead to larger AID and lower FMM at the most apical electrode. Due to the variations in the size of the cochlea, a preoperative determination of the CDL could be useful to investigate a possible influence of the anatomy-based selection of the electrode array on the period of acclimatization to CI-mediated hearing, the hearing quality for speech and music, as well as speech comprehension in noise. No significant correlations were found between FMM and FMT, nor between AID and FMT. Furthermore, in the cohort of patients studied, no significant difference in SU in quiet was found between cases with incomplete electrode insertion and deactivated electrodes on the one hand and fully inserted cases on the other. The results suggest that the FMM may not be as crucial for the SU of monosyllabic test words as previously thought.
5.1 Conclusions for practical application
- In order to achieve a specific insertion depth with regard to the insertion angle of the most apical electrode, it is recommended to determine the individual length of the cochlear duct.
- The results of the Freiburg monosyllabic test in quiet after one year of cochlear implant rehabilitation show no statistically reliable correlation with the frequency mismatch of the apical electrode channel.
5.2 Outlook
The findings of this study, in conjunction with those of previous investigations, indicate that variations in SU are attributable to patient-specific factors and are not predominantly attributed to the FMM. Future research should investigate the impact of FMM on the duration needed to attain optimal SU. Recently, Dessard et al. [8] demonstrated a direct correlation between the size of the FMM and the adaptation speed of the patients. It should be examined whether the anatomy-based selection of the electrode array contributes to a shorter time to achieve optimal auditory outcomes.
Notes
Ethics statement
The ethics committee of the Faculty of Medicine at J. W. Goethe University Frankfurt am Main gave a positive vote in favor of conducting the study (case number 2022-642).
Competing interests
The authors declare that they have no competing interests.
References
[1] Alexiades G, Dhanasingh A, Jolly C. Method to estimate the complete and two-turn cochlear duct length. Otol Neurotol. 2015 Jun;36(5):904-7. DOI: 10.1097/MAO.0000000000000620[2] Breitsprecher T, Mlynski R, Völter C, Van de Heyning P, Van Rompaey V, Dazert S, Weiss NM. Accuracy of Preoperative Cochlear Duct Length Estimation and Angular Insertion Depth Prediction. Otol Neurotol. 2023 Sep;44(8):e566-e571. DOI: 10.1097/MAO.0000000000003956
[3] Breitsprecher TM, Baumgartner WD, Brown K, Dazert S, Doyle U, Dhanasingh A, Großmann W, Hagen R, Van de Heyning P, Mlynski R, Neudert M, Rajan G, Rak K, Van Rompaey V, Schmutzhard J, Volkenstein S, Völter C, Wimmer W, Zernotti M, Weiss NM. Effect of Cochlear Implant Electrode Insertion Depth on Speech Perception Outcomes: A Systematic Review. Otol Neurotol Open. 2023 Dec;3(4):e045. DOI: 10.1097/ONO.0000000000000045
[4] Bruns L, Mürbe D, Hahne A. Understanding music with cochlear implants. Sci Rep. 2016 Aug;6:32026. DOI: 10.1038/srep32026
[5] Canfarotta MW, Dillon MT, Buss E, Pillsbury HC, Brown KD, O'Connell BP. Frequency-to-Place Mismatch: Characterizing Variability and the Influence on Speech Perception Outcomes in Cochlear Implant Recipients. Ear Hear. 2020;41(5):1349-61. DOI: 10.1097/AUD.0000000000000864
[6] Canfarotta MW, O'Connell BP, Giardina CK, Buss E, Brown KD, Dillon MT, Rooth MA, Pillsbury HC, Buchman CA, Adunka OF, Fitzpatrick DC. Relationship Between Electrocochleography, Angular Insertion Depth, and Cochlear Implant Speech Perception Outcomes. Ear Hear. 2021;42(4):941-8. DOI: 10.1097/AUD.0000000000000985
[7] Chakravorti S, Noble JH, Gifford RH, Dawant BM, O'Connell BP, Wang J, Labadie RF. Further Evidence of the Relationship Between Cochlear Implant Electrode Positioning and Hearing Outcomes. Otol Neurotol. 2019 Jun;40(5):617-24. DOI: 10.1097/MAO.0000000000002204
[8] Dessard L, Gersdorff G, Ivanovik N, Zoca-Assadi M, Nopp P, Camby S, Lefebvre PP. Cochlear Implant: Analysis of the Frequency-to-Place Mismatch with the Table-Based Software OTOPLAN® and Its Influence on Hearing Performance. Audiol Neurootol. 2024;29(3):239-45. DOI: 10.1159/000535693
[9] Dhanasingh A, Hochmair I. Signal processing & audio processors. Acta Otolaryngol. 2021 Mar;141(sup1):106-34. DOI: 10.1080/00016489.2021.1888504
[10] Drennan WR, Svirsky MA, Fitzgerald MB, Rubinstein JT. Mimicking normal auditory functions with cochlear implant sound processing; past, present and future. In: Waltzman SB, Roland JT, editors. Cochlear Implants. New York: Thieme; 2014.
[11] Dutrieux N, Quatre R, Péan V, Schmerber S. Correlation Between Cochlear Length, Insertion Angle, and Tonotopic Mismatch for MED-EL FLEX28 Electrode Arrays. Otol Neurotol. 2022 Jan;43(1):48-55. DOI: 10.1097/MAO.0000000000003337
[12] Escudé B, James C, Deguine O, Cochard N, Eter E, Fraysse B. The size of the cochlea and predictions of insertion depth angles for cochlear implant electrodes. Audiol Neurootol. 2006;11 Suppl 1:27-33. DOI: 10.1159/000095611
[13] Greenwood DD. Critical bandwidth and consonance: their operational definitions in relation to cochlear nonlinearity and combination tones. Hear Res. 1991 Aug;54(2):209-46. DOI: 10.1016/0378-5955(91)90118-s
[14] Greenwood DD. Critical bandwidth and the frequency coordinates of the basilar membrane. J Acoust Soc Am. 1961;33:1344-56. DOI: 10.1121/1.1908437
[15] Heutink F, Verbist BM, van der Woude WJ, Meulman TJ, Briaire JJ, Frijns JHM, Vart P, Mylanus EAM, Huinck WJ. Factors Influencing Speech Perception in Adults With a Cochlear Implant. Ear Hear. 2021;42(4):949-60. DOI: 10.1097/AUD.0000000000000988
[16] Ketterer MC, Aschendorff A, Arndt S, Speck I, Rauch AK, Beck R, Hassepass F. Radiological evaluation of a new straight electrode array compared to its precursors. Eur Arch Otorhinolaryngol. 2021 Oct;278(10):3707-14. DOI: 10.1007/s00405-020-06434-5
[17] Koch RW, Ladak HM, Elfarnawany M, Agrawal SK. Measuring Cochlear Duct Length - a historical analysis of methods and results. J Otolaryngol Head Neck Surg. 2017 Mar;46(1):19. DOI: 10.1186/s40463-017-0194-2
[18] Lambriks L, van Hoof M, Debruyne J, Janssen M, Chalupper J, van der Heijden K, Hof J, Hellingman K, Devocht E, George E. Imaging-based frequency mapping for cochlear implants - Evaluated using a daily randomized controlled trial. Front Neurosci. 2023;17:1119933. DOI: 10.3389/fnins.2023.1119933
[19] Landsberger DM, Svrakic M, Roland JT Jr, Svirsky M. The Relationship Between Insertion Angles, Default Frequency Allocations, and Spiral Ganglion Place Pitch in Cochlear Implants. Ear Hear. 2015;36(5):e207-13. DOI: 10.1097/AUD.0000000000000163
[20] Lenarz M, Sönmez H, Joseph G, Büchner A, Lenarz T. Long-term performance of cochlear implants in postlingually deafened adults. Otolaryngol Head Neck Surg. 2012 Jul;147(1):112-8. DOI: 10.1177/0194599812438041
[21] Li H, Helpard L, Ekeroot J, Rohani SA, Zhu N, Rask-Andersen H. Three-dimensional tonotopic mapping of the human cochlea based on synchrotron radiation phase-contrast imaging. Sci Rep. 2021;11:4437. DOI: 10.1038/s41598-021-83225-w
[22] MacPhail ME, Connell NT, Totten DJ, Gray MT, Pisoni D, Yates CW, Nelson RF. Speech Recognition Outcomes in Adults With Slim Straight and Slim Modiolar Cochlear Implant Electrode Arrays. Otolaryngol Head Neck Surg. 2022 May;166(5):943-50. DOI: 10.1177/01945998211036339
[23] McCormack A, Fortnum H. Why do people fitted with hearing aids not wear them? Int J Audiol. 2013 May;52(5):360-8. DOI: 10.3109/14992027.2013.769066
[24] McJunkin JL, Durakovic N, Herzog J, Buchman CA. Early Outcomes With a Slim, Modiolar Cochlear Implant Electrode Array. Otol Neurotol. 2018 Jan;39(1):e28-e33. DOI: 10.1097/MAO.0000000000001652
[25] Mertens G, Van de Heyning P, Vanderveken O, Topsakal V, Van Rompaey V. The smaller the frequency-to-place mismatch the better the hearing outcomes in cochlear implant recipients? Eur Arch Otorhinolaryngol. 2022 Apr;279(4):1875-83. DOI: 10.1007/s00405-021-06899-y
[26] Neumayer HL, Adel Y, Baumann U. Radiologische Bestimmung der Position von Cochlea-Implantat-Elektroden und deren Frequenz-Zuordnung nach unterschiedlichen Modellen [Radiological determination of the position of cochlear implant electrodes and their frequency assignment according to different models]. GMS Z Audiol Audiol Acoust. 2020;2:Doc02. DOI: 10.3205/zaud000006
[27] Peters JPM, Bennink E, van Zanten GA. Comparison of Place-versus-Pitch Mismatch between a Perimodiolar and Lateral Wall Cochlear Implant Electrode Array in Patients with Single-Sided Deafness and a Cochlear Implant. Audiol Neurootol. 2019;24(1):38-48. DOI: 10.1159/000499154
[28] Pietsch M, Schurzig D, Salcher R, Warnecke A, Erfurt P, Lenarz T, Kral A. Variations in microanatomy of the human modiolus require individualized cochlear implantation. Sci Rep. 2022 Mar;12(1):5047. DOI: 10.1038/s41598-022-08731-x
[29] Schindela E. Die Cochlea-Implantatversorgung Erwachsener der HNO-Abteilung im Klinikum Großhadern - Retrospektive Auswertung der Ergebnisse [PhD thesis]. Munich: Ludwig-Maximilians-Universität; 2006. DOI: 10.5282/edoc.5706
[30] Schroeder A. Bilaterale Cochlea Implantat-Versorgung-Einflussfaktoren und deren Auswirkung auf das postoperative Hörergebnis: eine retrospektive Studie [PhD thesis]. Frankfurt am Main: Johann Wolfgang Goethe-Universität; 2016.
[31] Stakhovskaya O, Sridhar D, Bonham BH, Leake PA. Frequency map for the human cochlear spiral ganglion: implications for cochlear implants. J Assoc Res Otolaryngol. 2007 Jun;8(2):220-33. DOI: 10.1007/s10162-007-0076-9
[32] Venail F, Mathiolon C, Menjot de Champfleur S, Piron JP, Sicard M, Villemus F, Vessigaud MA, Sterkers-Artieres F, Mondain M, Uziel A. Effects of electrode array length on frequency-place mismatch and speech perception with cochlear implants. Audiol Neurootol. 2015;20(2):102-11. DOI: 10.1159/000369333
[33] Videhult Pierre P, Eklöf M, Smeds H, Asp F. Cochlear Implantation with the CI512 and CI532 Precurved Electrode Arrays: One-Year Speech Recognition and Intraoperative Thresholds of Electrically Evoked Compound Action Potentials. Audiol Neurootol. 2019;24(6):299-308. DOI: 10.1159/000504592
[34] Zwolan TA, Basura G. Determining Cochlear Implant Candidacy in Adults: Limitations, Expansions, and Opportunities for Improvement. Semin Hear. 2021 Nov;42(4):331-41. DOI: 10.1055/s-0041-1739283
Attachments
| Attachment 1 | Appendix 1 and 2 (zaud000069_Attachment1.pdf, application/pdf, 217.46 KBytes) |




