[Bessere Versorgung von Kindern mit Appendizitis: durch die Einführung von Antibiotic Stewardship wird die postoperative Therapie optimiert]
Sebastian Beltz 1,2Stephanie Fischer 3
Frank Huenger 4
Reza Vahdad 5
Hermann Kalhoff 6
Andreas Leutner 1
1 Department of Pediatric Surgery and Pediatric Urology, Children’s Hospital, Klinikum Dortmund, Germany
2 University Witten/Herdecke, Witten, Germany
3 Department of Pharmacology, Klinikum Dortmund, Germany
4 Institute for Hospital Hygiene and Clinical Microbiology, Klinikum Dortmund, Germany
5 Department of Pediatric Surgery and Pediatric Urology, University Hospitals of Gießen and Marburg, Germany
6 Department of Pediatrics and Adolescent Medicine, Children’s Hospital, Klinikum Dortmund, Germany
Zusammenfassung
Ziel: Die Entfernung des Wurmfortsatzes ist die häufigste abdominelle Notfalloperation bei Kindern, die verhältnismäßig oft mit einer fortgeschrittenen Appendizitis vorstellig werden und daher eine zielgerichtete antibiotische Therapie benötigen. Während in anderen medizinischen Fachbereichen die Prinzipien des Antibiotic Stewardship (ABS) zunehmend etabliert sind, werden diese in der Kinderchirurgie immer noch nicht konsequent berücksichtigt. Ziel dieser Studie war es daher, die Auswirkungen der Implementierung einer ABS-konformen SOP auf die postoperative Versorgung dieser Patientengruppe zu untersuchen.
Material und Methoden: Wir verglichen die Qualität der peri- und postoperativen Antibiotikatherapie vor und nach der Implementierung einer Standardarbeitsanweisung (SOP) im Jahr 2020. Die Versorgung pädiatrischer Patienten, bei denen eine Appendektomie durchgeführt wurde, wurde anhand eines Algorithmus bewertet, der aus unserer Sicht die empfohlene Antibiotikatherapie gemäß aktueller Literatur und guter klinischer Praxis darstellt. Insgesamt wurden 165 Patientenfälle vor und 209 Patienten nach Einführung der SOP ausgewertet.
Ergebnisse: Nach Einführung der SOP wurde in durchschnittlich 10,5% weniger Fällen eine postoperative antibiotische Therapie initiiert (p-Wert 0,036). Die nach dem in diesem Rahmen entwickelten Score evaluierte Qualität der Behandlung verbesserte sich um 31,2% (p<0,0001). Die mediane Dauer der Antibiotikabehandlung bei fortgeschrittener Appendizitis war 2,0 Tage kürzer (p=0,062). Der Anteil der fortgeführten oralen Antibiotikabehandlung nach Entlassung sank um 25,6% (p<0,0001). Dabei wurde kein signifikanter Effekt auf die mediane Krankenhausverweildauer oder die Komplikationsrate beobachtet.
Schlussfolgerung: Die Implementierung einer auf den Prinzipien des ABS basierenden SOP hatte einen positiven Einfluss auf die Behandlungsqualität nach Appendektomie im Kindesalter. Der für diese Studie entwickelte Behandlungsalgorithmus kann zukünftigen Kinderchirurgen als Entscheidungshilfe für die Wahl der korrekten antimikrobiellen Therapie bei Appendizitis dienen.
Schlüsselwörter
Appendizitis im Kindesalter, postoperative Behandlung, Antibiotic Stewardship, Kinderchirurgie, verbesserter Outcome
Introduction
Acute appendicitis (AA), with a lifetime risk of 7–8% and a peak incidence in the early teens, is the most common reason for emergency treatment and surgery in pediatric patients with acute abdominal pain [1], [2]. Not only the incidence, but also the severity upon presentation at the surgical unit is higher in the population of low- and middle-income countries as well as in the younger population worldwide [3], [4]. While the proportion of complicated acute appendicitis (CAA) in adult patients is estimated to be around 30%, literature shows a much higher proportion of advanced disease, perforation and/or abscess formation in the pediatric population, for various reasons [3], [5], [6].
In the absence of a precise definition of what characterizes CAA as opposed to non-complicated appendicitis (NCA), a common basis for comparison is still lacking [7]. Nevertheless, cases of CAA or even organ perforation in children certainly require careful intraoperative assessment and postoperative care, including effective antibiotic treatment, as they are associated with an increased likelihood of postoperative complications such as surgical site infection (SSI) or abscess formation. To prevent these complications, optimal antibiotic therapy after appendectomy has been the subject of scientific attention for years, but a consistent approach has yet to be found. The principles of antibiotic stewardship (ABS), which aim to standardize and improve anti-inflammatory treatment, have been widely implemented in adult and pediatric medicine [8], [9], [10], [11]. However, several publications indicate a lack of knowledge about the aspects of anti-inflammatory treatment and prophylaxis within the pediatric surgical community [12], [13], [14], [15].
Certainly, it is indisputable that young patients suffering from appendicitis at all stages deserve optimal treatment based on professional microbiological assessment in the context of intraoperative findings, expected pathogens and their possible antimicrobial resistance. Therefore, we aimed to optimize our clinical approach to postoperative antibiotic management by introducing a standardized pathway for all stages of appendicitis. By combining Good Clinical Practice (GCP) with the principles of ABS, we hypothesized that we could regulate antibiotic use more efficiently to improve postoperative management of children.
Figure 1 [Fig. 1] provides a graphical abstract of the results obtained in this manner.
Figure 1: Optimizing postoperative treatment of pediatric appendicitis with ABS-compliant SOP; a single-center retrospectove analysis of the impact of the implementation of an antibiotic stewardship (ABS)-compliant standard operating procedure (SOP) on the quality of postoperative antibiotic treatment in 386 children undergoing appendectomy.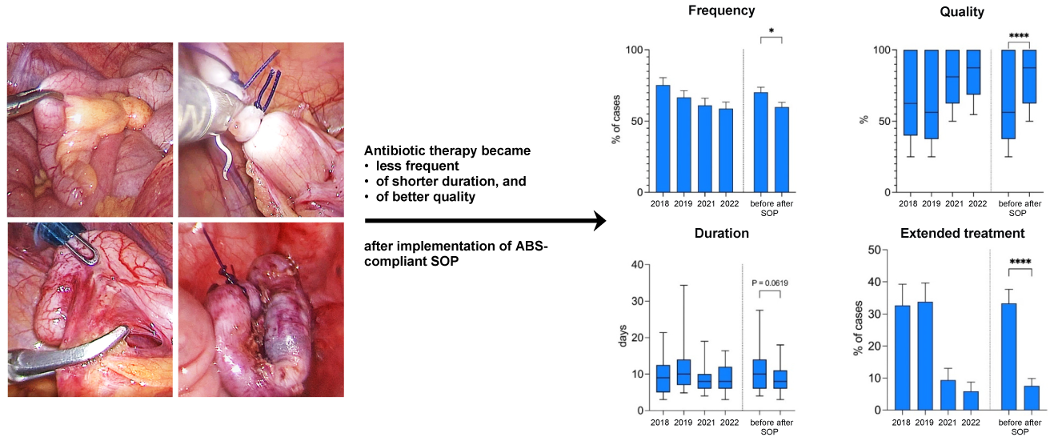
Material and methods
In the summer of 2020, we implemented a standard operating procedure (SOP) for peri/postoperative antibiotic regimens based on intraoperative findings: Patients with non-complicated appendicitis (NCA) should not receive postoperative antibiotic therapy in addition to the mandatory preoperative single-shot Cefuroxime. Patients with complicated acute appendicitis (CAA) were to receive postoperative Cefuroxime/Metronidazole, and in cases of perforation or intra-abdominal abscess (IAA), Piperacillin/Tazobactam was demanded to cover expected pathogens [16], [17], [18], [19], [20]. NCA was defined by the absence of peritonitis, ulceration, and gangrenous inflammation. CAA with these features was distinguished from perforation or abscess formation because different antibiotic regimens were required. The SOP was the subject of presentations at institutional journal clubs throughout 2020 and was made available on the clinic’s intranet for permanent access. Contact with the ABS team as well as the departments of clinical microbiology, infectious disease, and pharmacy was provided for any questions that arose.
To investigate surgeons’ compliance and detect differences in outcomes, we performed a single-center retrospective analysis of all children (0–15 years) who underwent appendectomy coded according to ICD-10 (K35.XX) between 2018 and 2022. Patients were assigned to either “before” (2018/2019) or “after” (2021/2022) the introduction of this ABS-compliant peri-/postoperative antibiotic regimen in the summer of 2020. The entire year 2020 was considered a transition period and therefore excluded from the final comparison. Of the total of 435 patients treated for appendicitis in 2018, 2019, 2021, and 2022, 49 were excluded from the study because they were miscoded as AA, were treated conservatively, or underwent elective appendectomy for another reason (e.g., interval appendectomy after acute inflammation). Of the remaining 386 cases, digital and/or analog charts, surgical documentation, as well as microbiological and histological findings were reviewed to evaluate defined end points (length of stay [LOS], complication rate, adequate implementation and duration of postoperative antibiotic therapy, correct antibiotic agent used, adequate and on time de-escalation and oralization, length of antibiotic therapy [LAT], and appropriate escalation of antibiotic therapy if indicated).
Our algorithm depicting the recommended treatment after appendectomy was developed using the implemented SOP, current scientific evidence [8], [21], [22], [23], [24], [25], [26], [27], [28], [29], and good clinical practice (GCP) as reviewed by the heads of the departments of pediatric infectiology, pharmacology, clinical microbiology, and pediatric surgery (Figure 2 [Fig. 2]).
Figure 2: Postoperative management after appendectomy, with consideration of intraoperative findings and frequent clinical evaluation according to scientific evidence and good clinical practice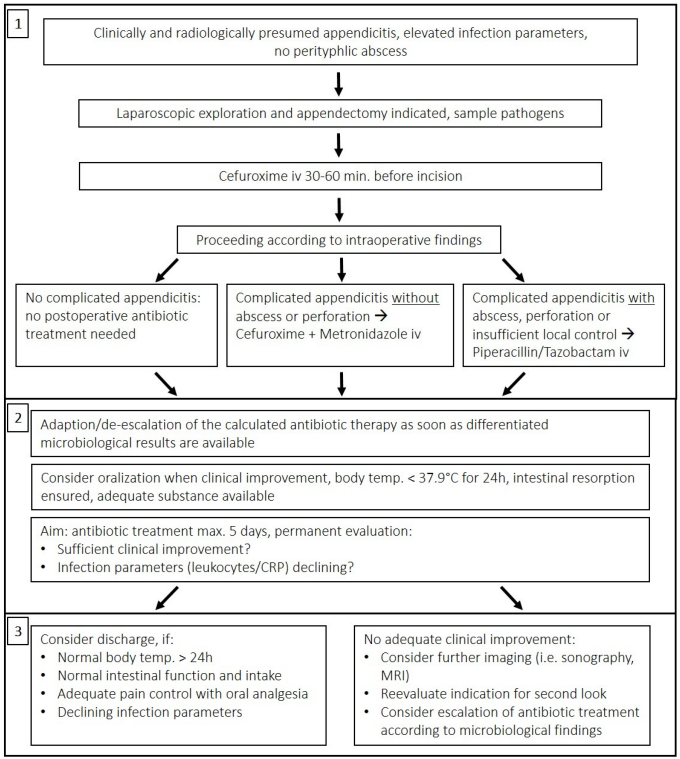
For evaluation, we developed a scoring system that awarded points for correct decision-making at key branching points following the algorithm. These marked either a significant change in patient status (e.g., surgery, discharge) or findings that required a specific action (based on the intraoperative macroscopic aspect, postoperative clinical course, laboratory or microbiological parameters). A maximum of 20 points (=100% quality of antibiotic therapy [QOT]) was awarded for correct therapeutic decisions throughout the course. In addition, points were deducted for serious deviations from the key principles of ABS. Patients’ postoperative antibiotic regimens were evaluated individually and independently by two reviewers who were considered experienced in antimicrobial treatment and ABS and who, in most cases, were not involved in the initial surgical decision. A discrepancy of more than 2 points (10%) within the independent assessment required comparison and mutual reconsideration. For reviewers and interested readers, we provide additional information on the structure and approach of this assessment in Attachment 1 [Att. 1]).
Statistical analysis was performed using Excel (version 16.8) and Graphpad Prism (version 10). The 2018/2019 and 2021/2022 groups largely reflect a similar distribution in terms of disease severity and complication and conversion rates, as shown in Table 1 [Tab. 1]. Therefore, we consider the groups to be essentially comparable.
Table 1: Severity of acute appendicitis as macroscopically described at the time of surgery, conversion, and primary complications before (2018/2019) and after (2021/2022) implementation of a standard operating procedure (SOP) in 2020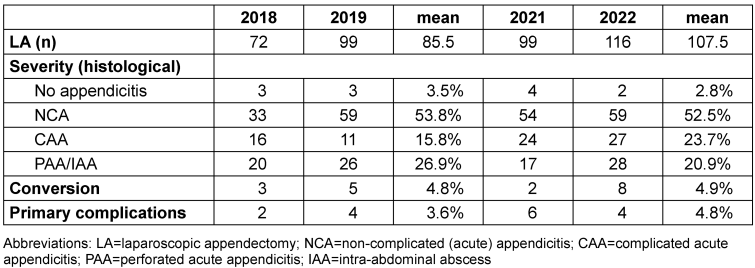
A Gaussian distribution was tested and excluded in all samples with regular normality tests (Shapiro-Wilk, Kolmogorov-Smirnov, QQ-Plot). Therefore, statistical significance was tested using the Mann-Whitney test, with p-values <0.05 defined as statistically significant. Bars show mean and standard error; box-and-whisker plots show median and 95% confidence interval.
Results
In our central European tertiary care pediatric surgery unit, we performed an average of 97 laparoscopic appendectomies per year between 2018 and 2022. 40–50% of the patients included here presented with advanced stage appendicitis (CAA), with approximately ¼ already macroscopically perforated (PAA) or presenting with intra-abdominal abscess (IAA) at the time of surgery (Table 1 [Tab. 1]). A chi-squared test of the means of 2018/2019 vs. 2021/2022 showed no significant difference in the distribution of disease severity, conversion rate, and complication rate (p-value 0.9108).
From 2018/2019 to 2021/2022, the number of cases in which postoperative antibiotic therapy was administered decreased by 10.5%, from 70.3% to 59.8% (p-value 0.036). This was primarily due to a decrease in postoperative therapy in the NCA group from 50.5% of cases in 2018/19 to 34.2% of cases in 2021/22, while the indication for postoperative antibiotic therapy in cases of advanced stage appendicitis (CAA/PAA/IAA) showed a stable proportion of 91.9% in 2018/19 and 88.2% in 2021/22 (Figure 3 [Fig. 3]).
Figure 3: Percentage of cases receiving postoperative antibiotic treatment after appendectomy and quality of the antibiotic treatment before and after the implementation of a standardized antibiotic regimen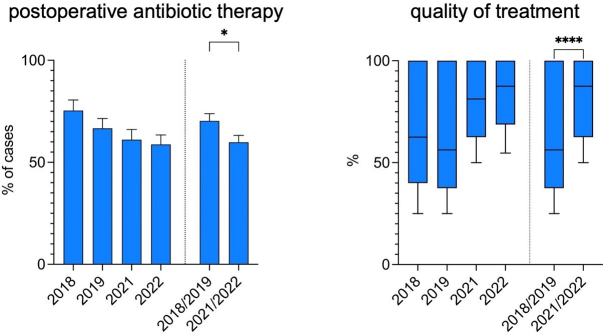
The quality of this antibiotic treatment in terms of the principles of ABS and GCP was significantly improved. While the median overall quality of antibiotic therapy (QOT) was 56.3% in 2018/2019, it reached 87.5% in 2021/2022 (p-value <0.0001) with an upward trend over time correlating with the therapists’ increasing compliance with following the protocol.
The median length of the antibiotic therapy (LAT) after appendectomy for advanced stage appendicitis was also reduced by 2 days overall (10 days in 2018/2019 vs. 8 days in 2021/2022, p-value 0.062). In addition, the mean rate of (extended) oral antibiotic treatment after discharge was lowered from 33.3% in 2018/2019 to 7.6% in 2021/2022 (p-value <0.0001) (Figure 4 [Fig. 4]).
Figure 4: Duration of antibiotic treatment after appendectomy for complicated appendicitis and percentage of cases with (prolonged) oral antibiotic treatment after discharge before and after implementation of a standardized antibiotic regimen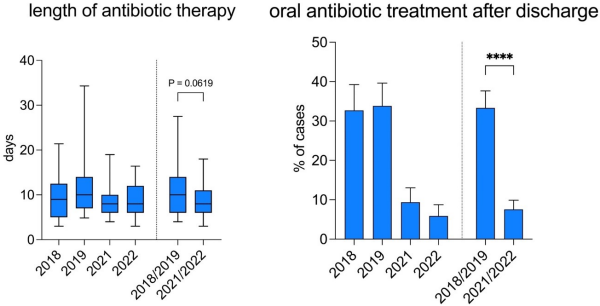
There was no relevant change in overall median length of the hospital stay (LOS) after appendectomy (5 days 2018/2019 vs. 5 days 2021/2022, p-value 0.2208) or in the number of primary postoperative complications, such as SSI or secondary intra-abdominal abscess formation (Table 1 [Tab. 1]).
Discussion
SOPs improve the effectiveness of ABS programs and the appropriateness of antibiotic regimens [30], [31]. However, although there are occasional reports of improved outcomes and minimization of postoperative complications with the implementation of an SOP after appendectomy in pediatric surgery, robust data remain scarce [32], [33], [34]. This is largely due to the fact that studies in pediatric surgery have primarily focused on the comparison of drug regimens within individual stages of AA [35], [36], [37]. Also, a clear differentiation of the intraoperative macroscopic findings, especially what objectively defines complicated acute appendicitis, has not yet been found. Therefore, a valid comparison of these studies is difficult [7], [38]. In addition, health care resources and structures vary widely between regions of the world, making it even more difficult to compare scientific findings and convert them into local GCP. As a result, common recommendations are based on limited evidence and are often at odds with common practice [39]. Thus, inter-surgeon agreement is poor with respect to subjective appendicitis classification and objective utilization of postoperative antibiotics [40]. In Europe, for example, therapeutic approaches vary widely from one institution to another [41]. To our knowledge, for these and other reasons, there is no scientifically proven, published SOP that condenses an optimal approach defining and reflecting all stages of AA in the context of healthcare resources in Europe.
The aim of this study was to evaluate how the implementation of an SOP, considering intraoperative findings, local microbial conditions and GCP, influences the QOT for pediatric patients with AA of all stages after primary LA. The underlying algorithm was developed with respect to existing published protocols and previous literature with the goal of finding the optimal therapy to prevent SSI or postoperative abscess formation, shorten the length of hospital stays and antibiotic treatment as responsibly as possible, and cover the expected spectrum of pathogens with the initially calculated antibiotic regimen (see also Attachment 1 [Att. 1]) [16], [35], [37], [42], [43], [44], [45], [46], [47].
First, this analysis shows that the implementation of standardized ABS-compliant postoperative antibiotic treatment based on intraoperative findings and surgeon’s assessment in cases of acute appendicitis was associated with improvements in the postoperative care of affected children in terms of quality and duration of antibiotic therapy. This did not increase the incidence of primary complications or prolong inpatient treatment to any relevant extent.
Second, to assess quality of treatment, we introduced an algorithm for retrospective evaluation of current institutional practice versus “ideal care”, as shown in Figure 2 [Fig. 2]. This approach may be a useful tool that could be adapted and then potentially used for evaluation in other circumstances to assess ABS compliance under numerous typical surgical circumstances requiring anti-infective treatment. Consequently, it may help to further implement the principles of ABS in pediatric surgery.
Limitations
The algorithm presented here is based on the current evidence for antibiotic therapy after laparoscopic appendectomy given different stages of acute appendicitis and the microbial distribution as reviewed in the contemporary literature, with the limitations mentioned above. Further research is needed to elucidate the evidence for antimicrobial therapy in pediatric acute appendicitis.
Although we were able to show that standardization of postoperative therapy is associated with a reduction in the duration of such therapy, the decision of when to discharge and when to discontinue antibiotic therapy is typically influenced not only by objective criteria, but also by the experience, understanding of safety, and personal attitude of the surgeon in charge, who in turn is influenced by his or her training as well as the “local culture” in a specific clinical setting. This aspect must be considered in the interpretation of the present results and in the design of further studies, as it may be a source of bias. For instance, the effect of implementing objective discharge criteria, as shown in Figure 2 [Fig. 2] (Section 3), on treatment efficacy and outcome may be of interest for further investigation.
Lastly, this study has the inherent limitations of a single-center retrospective analysis. Therefore, further prospective multicenter studies are needed.
Conclusion
The implementation of SOP for peri- and postoperative care after appendectomy, considering the principles of ABS, was associated with an improvement in the quality of postoperative antibiotic treatment in pediatric patients, especially in cases of advanced stage appendicitis – as summarized in the graphical abstract. This was demonstrated using a novel algorithm to evaluate the current QOT compared to the standards of ABS in acute appendicitis. In general, ABS models should be better adapted to pediatric surgical procedures in order to develop acceptable implementation strategies that further optimize antibiotic prescribing in pediatric surgery.
Notes
Conflict of interest
The authors declare that they have no competing interests.
Further acknowledgments
None.
Funding
No external financial support has been provided.
References
[1] Stewart B, Khanduri P, McCord C, Ohene-Yeboah M, Uranues S, Vega Rivera F, Mock C. Global disease burden of conditions requiring emergency surgery. Br J Surg. 2014 Jan;101(1):e9-22. DOI: 10.1002/bjs.9329[2] Ferris M, Quan S, Kaplan BS, Molodecky N, Ball CG, Chernoff GW, Bhala N, Ghosh S, Dixon E, Ng S, Kaplan GG. The Global Incidence of Appendicitis: A Systematic Review of Population-based Studies. Ann Surg. 2017 Aug;266(2):237-41. DOI: 10.1097/SLA.0000000000002188
[3] Kong VY, Sartorius B, Clarke DL. Acute appendicitis in the developing world is a morbid disease. Ann R Coll Surg Engl. 2015 Jul;97(5):390-5. DOI: 10.1308/003588415X14181254790608
[4] Körner H, Söndenaa K, Söreide JA, Andersen E, Nysted A, Lende TH, Kjellevold KH. Incidence of acute nonperforated and perforated appendicitis: age-specific and sex-specific analysis. World J Surg. 1997;21(3):313-7. DOI: 10.1007/s002689900235
[5] Abbas A, Laverde R, Yap A, Stephens CQ, Samad L, Seyi-Olajide JO, Ameh EA, Ozgediz D, Lakhoo K, Bickler SW, Meara JG, Bundy D, Jamison DT, Klazura G, Sykes A, Philipo GS; GICS. Routine Pediatric Surgical Emergencies: Incidence, Morbidity, and Mortality During the 1st 8000 Days of Life-A Narrative Review. World J Surg. 2023 Dec;47(12):3419-28. DOI: 10.1007/s00268-023-07097-z
[6] Baxter KJ, Nguyen HTMH, Wulkan ML, Raval MV. Association of Health Care Utilization With Rates of Perforated Appendicitis in Children 18 Years or Younger. JAMA Surg. 2018 Jun;153(6):544-50. DOI: 10.1001/jamasurg.2017.5316
[7] Kwok CPD, Tsui SYB, Chan KWE. Updates on bacterial resistance and empirical antibiotics treatment of complicated acute appendicitis in children. J Pediatr Surg. 2021 Jul;56(7):1145-9. DOI: 10.1016/j.jpedsurg.2021.03.027
[8] Abam R, Bielicki J, Brinkmann F, Dedy J, Gille C, Groll A, et al. S2k Leitlinie "Antibiotic Stewardship - Konzeption und Umsetzung in derstationären Kinder- und Jugendmedizin" - Version 1.12.2018. AWMF-Registernummer 048/15. AWMF;2018.
[9] Newland JG, Hersh AL. Purpose and design of antimicrobial stewardship programs in pediatrics. Pediatr Infect Dis J. 2010 Sep;29(9):862-3. DOI: 10.1097/INF.0b013e3181ef2507
[10] Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, Srinivasan A, Dellit TH, Falck-Ytter YT, Fishman NO, Hamilton CW, Jenkins TC, Lipsett PA, Malani PN, May LS, Moran GJ, Neuhauser MM, Newland JG, Ohl CA, Samore MH, Seo SK, Trivedi KK. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016 May;62(10):e51-77. DOI: 10.1093/cid/ciw118
[11] Gerber JS, Jackson MA, Tamma PD, Zaoutis TE; COMMITTEE ON INFECTIOUS DISEASES, PEDIATRIC INFECTIOUS DISEASES SOCIETY. Antibiotic Stewardship in Pediatrics. Pediatrics. 2021 Jan;147(1):e2020040295. DOI: 10.1542/peds.2020-040295
[12] Williams K, Baumann L, Abdullah F, St Peter SD, Oyetunji TA. Variation in prophylactic antibiotic use for laparoscopic cholecystectomy: need for better stewardship in pediatric surgery. J Pediatr Surg. 2017 Oct 9:S0022-3468(17)30634-6. DOI: 10.1016/j.jpedsurg.2017.10.012
[13] Malone SM, Seigel NS, Newland JG, Saito JM, McKay VR. Understanding antibiotic prophylaxis prescribing in pediatric surgical specialties. Infect Control Hosp Epidemiol. 2020 Jun;41(6):666-71. DOI: 10.1017/ice.2020.71
[14] Anandalwar SP, Milliren C, Graham DA, Hills-Dunlap JL, Kashtan MA, Newland J, Rangel SJ. Trends in the use of surgical antibiotic prophylaxis in general pediatric surgery: Are we missing the mark for both stewardship and infection prevention? J Pediatr Surg. 2020 Jan;55(1):75-9. DOI: 10.1016/j.jpedsurg.2019.09.057
[15] Kim JK, Chua ME, Ming JM, Braga LH, Smith GHH, Driver C, Koyle MA. Practice variation on use of antibiotics: An international survey among pediatric urologists. J Pediatr Urol. 2018 Dec;14(6):520-4. DOI: 10.1016/j.jpurol.2018.04.018
[16] Martin B, Subramanian T, Arul S, Patel M, Jester I. Using Microbiology Culture in Pediatric Appendicitis to Risk Stratify Patients: A Cohort Study. Surg Infect (Larchmt). 2023 Mar;24(2):183-9. DOI: 10.1089/sur.2022.220
[17] Naji H, Jayakumar J, Ali R. Bacterial profile and antibiotic selection for pediatric appendicitis: A retrospective cohort study. Surg Open Sci. 2023 Aug;14:120-3. DOI: 10.1016/j.sopen.2023.07.018
[18] Bhaskar K, Clarke S, Moore LSP, Hughes S. Bacterial peritonitis in paediatric appendicitis; microbial epidemiology and antimicrobial management. Ann Clin Microbiol Antimicrob. 2023 Jun;22(1):45. DOI: 10.1186/s12941-023-00591-1
[19] Theodorou CM, Stokes SC, Hegazi MS, Brown EG, Saadai P. Is Pseudomonas infection associated with worse outcomes in pediatric perforated appendicitis? J Pediatr Surg. 2021 Oct;56(10):1826-30. DOI: 10.1016/j.jpedsurg.2020.10.031
[20] Hu A, Li J, Vacek J, Bouchard M, Ingram MC, McMahon M, Mithal LB, Raval MV, Reynolds M, Goldstein S. Antibiotic resistance is common in the cultures of intraabdominal abscess drainage after appendectomy. J Pediatr Surg. 2022 Sep;57(9):102-6. DOI: 10.1016/j.jpedsurg.2021.12.003
[21] Andric M, Kalff JC, Schwenk W, Farkas S, Hartwig W, Türler A, et al. Empfehlungen zur Therapie der akuten Appendizitis. AWMF-Register-Nr 088-011, Klassifikation S1. AWMF;2021.
[22] de With K, Wilke K, Kern W V, Richard Strauß P, Kramme E, Friedrichs A, et al. S3-Leitlinie "Strategien zur Sicherung rationaler Antibiotika-Anwendung im Krankenhaus." AWMF-Registernummer 092/001 - Update 2018. AWMF;2018.
[23] van Rossem CC, Schreinemacher MH, Treskes K, van Hogezand RM, van Geloven AA. Duration of antibiotic treatment after appendicectomy for acute complicated appendicitis. Br J Surg. 2014 May;101(6):715-9. DOI: 10.1002/bjs.9481
[24] Snelling CM, Poenaru D, Drover JW. Minimum postoperative antibiotic duration in advanced appendicitis in children: a review. Pediatr Surg Int. 2004 Dec;20(11-12):838-45. DOI: 10.1007/s00383-004-1280-x
[25] Ketha B, Stephenson KJ, Dassinger MS 3rd, Smith SD, Burford JM. Eliminating Use of Home Oral Antibiotics in Pediatric Complicated Appendicitis. J Surg Res. 2021 Jul;263:151-4. DOI: 10.1016/j.jss.2020.12.059
[26] Hall NJ, Kapadia MZ, Eaton S, Chan WW, Nickel C, Pierro A, Offringa M. Outcome reporting in randomised controlled trials and meta-analyses of appendicitis treatments in children: a systematic review. Trials. 2015 Jun;16:275. DOI: 10.1186/s13063-015-0783-1
[27] Andersen BR, Kallehave FLFLK, Andersen HK. Antibiotics versus placebo for prevention of postoperative infection after appendicectomy. Cochrane Database Syst Rev. 2003;(2): CD001439. DOI: 10.1002/14651858.CD001439.
[28] Blot S, De Waele JJ, Vogelaers D. Essentials for selecting antimicrobial therapy for intra-abdominal infections. Drugs. 2012 Apr;72(6):e17-32. DOI: 10.2165/11599800-000000000-00000
[29] Lee SL, Islam S, Cassidy LD, Abdullah F, Arca MJ; 2010 American Pediatric Surgical Association Outcomes and Clinical Trials Committee. Antibiotics and appendicitis in the pediatric population: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J Pediatr Surg. 2010 Nov;45(11):2181-5. DOI: 10.1016/j.jpedsurg.2010.06.038
[30] Donà D, Luise D, Barbieri E, Masiero N, Maita S, Antoniello L, Zaoutis T, Giaquinto C, Gamba P. Effectiveness and Sustainability of an Antimicrobial Stewardship Program for Perioperative Prophylaxis in Pediatric Surgery. Pathogens. 2020 Jun 19;9(6):490. DOI: 10.3390/pathogens9060490
[31] Donà D, Luise D, La Pergola E, Montemezzo G, Frigo A, Lundin R, Zaoutis T, Gamba P, Giaquinto C. Effects of an antimicrobial stewardship intervention on perioperative antibiotic prophylaxis in pediatrics. Antimicrob Resist Infect Control. 2019;8:13. DOI: 10.1186/s13756-019-0464-z
[32] Laituri C, Arnold MA. A standardized guideline for antibiotic prophylaxis in surgical neonates. Semin Pediatr Surg. 2019 Feb;28(1):53-6. DOI: 10.1053/j.sempedsurg.2019.01.009
[33] Wakeman D, Livingston MH, Levatino E, Juviler P, Gleason C, Tesini B, Wilson NA, Pegoli W Jr, Arca MJ. Reduction of surgical site infections in pediatric patients with complicated appendicitis: Utilization of antibiotic stewardship principles and quality improvement methodology. J Pediatr Surg. 2022 Jan;57(1):63-73. DOI: 10.1016/j.jpedsurg.2021.09.031
[34] Khan S, Siow VS, Lewis A, Butler G, Narr M, Srinivasan S, Michaels M, Mollen K. An Evidence-Based Care Protocol Improves Outcomes and Decreases Cost in Pediatric Appendicitis. J Surg Res. 2020 Dec;256:390-6. DOI: 10.1016/j.jss.2020.05.067
[35] Lee J, Garvey EM, Bundrant N, Hargis-Villanueva A, Kang P, Osuchukwu O, Dekonenko C, Svetanoff WJ, St Peter SD, Padilla B, Ostlie D. IMPPACT (Intravenous Monotherapy for Postoperative Perforated Appendicitis in Children Trial): Randomized Clinical Trial of Monotherapy Versus Multi-drug Antibiotic Therapy. Ann Surg. 2021 Sep;274(3):406-10. DOI: 10.1097/SLA.0000000000005006
[36] Hamdy RF, Handy LK, Spyridakis E, Dona D, Bryan M, Collins JL, Gerber JS. Comparative Effectiveness of Ceftriaxone plus Metronidazole versus Anti-Pseudomonal Antibiotics for Perforated Appendicitis in Children. Surg Infect (Larchmt). 2019 Jul;20(5):399-405. DOI: 10.1089/sur.2018.234
[37] Robinson JR, Avritscher EBC, Gay JC, Willis ZI, Putnam LR, Anglemyer A, Pedroza C, Tyson JE, Blakely ML. Measuring the Value of a Clinical Practice Guideline for Children With Perforated Appendicitis. Ann Surg. 2017 Jul;266(1):195-200. DOI: 10.1097/SLA.0000000000001946
[38] Cramm SL, Lipskar AM, Graham DA, Kunisaki SM, Griggs CL, Allukian M, Russell RT, Chandler NM, Santore MT, Aronowitz DI, Blakely ML, Campbell B, Collins DT, Commander SJ, Cowles RA, DeFazio JR, Echols JC, Esparaz JR, Feng C, Guyer RA, Hanna DN, He K, Kahan AM, Keane OA, Lamoshi A, Lopez CM, McLean SE, Pace E, Regan MD, Scholz S, Tracy ET, Williams SA, Zhang L, Rangel SJ; Eastern Pediatric Surgery Network. Association of Gangrenous, Suppurative, and Exudative Findings With Outcomes and Resource Utilization in Children With Nonperforated Appendicitis. JAMA Surg. 2022 Aug;157(8):685-92. DOI: 10.1001/jamasurg.2022.1928
[39] Ferguson DM, Parker TD, Arshad SA, Garcia EI, Hebballi NB, Tsao K. Standardized Discharge Antibiotics May Reduce Readmissions in Pediatric Perforated Appendicitis. J Surg Res. 2020 Nov;255:388-95. DOI: 10.1016/j.jss.2020.05.086
[40] Buonpane CL, Vacek J, Harris CJ, Salazar Osuna JH, Van Arendonk KJ, Hunter CJ, Goldstein SD. Controversy in the classification of appendicitis and utilization of postoperative antibiotics. Surgery. 2022 Apr;171(4):1022-6. DOI: 10.1016/j.surg.2021.10.006
[41] Dotlacil V, Frybova B, Polívka N, Kardos D, Vajda P, Toczewski K, Pechanová R, Babala J, Rygl M, Patkowski D. Current management of pediatric appendicitis: A Central European survey. Adv Clin Exp Med. 2020 Jun;29(6):745-50. DOI: 10.17219/acem/122176
[42] Anderson KT, Bartz-Kurycki MA, Tsao K. Utility of standardized discharge criteria after appendectomy to identify pediatric patients requiring intervention after postoperative imaging. Surgery. 2018 Dec;164(6):1204-8. DOI: 10.1016/j.surg.2018.05.027
[43] Liu Q, Hao F, Chen B, Li L, Liu Q, Guo C. Multi-Center Prospective Study of Restrictive Post-Operative Antibiotic Treatment of Children with Complicated Appendicitis. Surg Infect (Larchmt). 2020 Nov;21(9):778-83. DOI: 10.1089/sur.2019.293
[44] Gordon AJ, Choi JH, Ginsburg H, Kuenzler K, Fisher J, Tomita S. Oral Antibiotics and Abscess Formation After Appendectomy for Perforated Appendicitis in Children. J Surg Res. 2020 Dec;256:56-60. DOI: 10.1016/j.jss.2020.05.082
[45] Lansdale N, Fryer S, Stockdale M, Bancroft J, Orr J, Corbett H, Kenny S. Prospective evaluation of a clinical response directed pathway for complicated appendicitis. J Pediatr Surg. 2019 Feb;54(2):272-5. DOI: 10.1016/j.jpedsurg.2018.10.082
[46] Somers KK, Eastwood D, Liu Y, Arca MJ. Splitting hairs and challenging guidelines: Defining the role of perioperative antibiotics in pediatric appendicitis patients. J Pediatr Surg. 2020 Mar;55(3):406-13. DOI: 10.1016/j.jpedsurg.2019.07.004
[47] Jen J, Hwang R, Mattei P. Post-discharge antibiotics do not prevent intra-abdominal abscesses after appendectomy in children. J Pediatr Surg. 2023 Feb;58(2):258-62. DOI: 10.1016/j.jpedsurg.2022.10.024
Attachments
| Attachment 1 | Additional information on the structure and approach of the assessment (Attachment1_dgkh000535.pdf, application/pdf, 72.85 KBytes) |




