[Kutane Lyme-Borreliose: Leitlinie der Deutschen Dermatologischen Gesellschaft]
Heidelore Hofmann 1Volker Fingerle 2
Sebastian Rauer 3
Klaus-Peter Hunfeld 4
Hans-Iko Huppertz 5
Andreas Krause 6
Consensus group
1 Klinik für Dermatologie und Allergologie der TU München, Munich, Germany
2 Nationales Referenzzentrum für Borrelien, Bayerisches Landesamt für Gesundheit und Lebensmittelsicherheit (LGL), Oberschleißheim, Germany
3 Neurologische Universitätsklinik, Freiburg, Germany
4 Zentralinstitut für Labormedizin, Mikrobiologie & Krankenhaushygiene, Krankenhaus Nordwest, Frankfurt, Germany
5 Professor-Hess-Kinderklinik Klinikum Bremen-Mitte, Bremen, Germany
6 Immanuel Krankenhaus Berlin, Germany
Zusammenfassung
Die aktuelle S2k-Leitlinie „kutane Borreliose“ wurde entsprechend den methodischen Standards der „Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V.“ aktualisiert. Sie wurde interdisziplinär von 18 AWMF-Mitgliedsgesellschaften, dem Robert Koch-Institut, der „Paul-Ehrlich-Gesellschaft für Infektionstherapie“, der „Instand e.V. – Gesellschaft zur Förderung der Qualitätssicherung in medizinischen Laboratorien“, der „Deutschen Borreliose-Gesellschaft“ und den beiden deutschen Patientenorganisationen „Borreliose und FSME Bund Deutschland“ und „Aktionsbündnis gegen zeckenübertragene Infektionen Deutschland e.V.“ konsentiert.
Diese Leitlinie der Deutschen Dermatologischen Gesellschaft befasst sich primär mit der Diagnostik und Therapie kutaner Manifestationen der Lyme-Borreliose und richtet sich an niedergelassene Ärzte und Kliniker, die Lyme-Borreliose behandeln. Ziele dieser Leitlinie sind Empfehlungen zur Sicherung der klinischen Diagnose, Empfehlungen zur stadienbezogenen Labordiagnostik sowie Empfehlungen zur Therapie der verschiedenen Manifestationen Erythema migrans, Multiple Erythemata migrantia, Borrelien-Lymphozytom und Acrodermatitis chronica atrophicans.
Die vorliegende Aktualisierung der Leitlinie berücksichtigt die internationale Literatur zu kutanen Manifestationen der Lyme-Borreliose bis 2022. Es gab keine wesentlichen Änderungen in Diagnostik und Therapie. Die Cochrane-Netzwerkanalyse zur Behandlung des Erythema migrans hat lediglich gezeigt, dass orales Penicillin V neben Doxycyclin und Amoxicillin ebenso wirksam ist. Eine slowenische Analyse von Patienten mit Acrodermatitis chronica atrophicans über die letzten 30 Jahre zeigte einen Rückgang der Inzidenz von Allgemeinsymptomen und Atrophie, vermutlich aufgrund einer verbesserten Früherkennung. Darüber hinaus ist der Leitlinie ein Informationsblatt für Patienten mit Empfehlungen zur Prävention der Lyme-Borreliose beigefügt.
Preamble
This guideline primarily focuses on the diagnosis and treatment of cutaneous manifestations of Lyme borreliosis.
It was approved in 2016 by 22 interdisciplinary medical societies and 2 patient organisations. Since then, a systematic literature review and evaluation has been conducted and published by the German Cochrane Centre, Freiburg (Cochrane Germany) for the further development to stage S3 for the treatment of erythema migrans [1].
In 2018, the same interdisciplinary guideline group published the guideline ‘Neuroborreliosis’, AWMF register number 030-071, development stage S3 [2].
What’s new?
The updated version of this guideline incorporates international publications on cutaneous manifestations of Lyme borreliosis up to 2022.
There have been no significant changes regarding diagnosis and treatment. Cochrane’s network meta-analysis on the treatment of erythema migrans has shown that oral penicillin V is just as effective as doxycycline and amoxicillin. A Slovenian study of patients with acrodermatitis chronica atrophicans, carried out over the last 30 years, has found that constitutional symptoms and atrophy are becoming less frequent [3], most likely due to improvements in early detection.
Synonyms
Cutaneous borreliosis, cutaneous manifestations of Lyme borreliosis, skin borreliosis, cutaneous Lyme borreliosis, cutaneous Lyme disease
Search terms
Borrelia burgdorferi infection, hard-bodied tick borreliosis, Lyme disease, cutaneous Lyme borreliosis, erythema migrans disease, erythema migrans, erythema chronicum migrans, lymphadenosis cutis benigna, borrelial lymphocytoma, multiple erythemata migrantia, multiple erythema migrans, acrodermatitis chronica atrophicans
ICD-10-No.: A69.2, L90.4
AWMF Guideline Register No. 013/044
Development stage: S2k
List of abbreviations
- ACA: Acrodermatitis chronica atrophicans
- BL: Borrelial lymphocytoma
- DD: Differential diagnosis
- d: Day
- EM: Erythema migrans
- ECM: Erythema chronicum migrans
- i.v.: Intravenous
- BW: Body weight
- LB: Lyme borreliosis
- LTT: Lymphozyte transformation test
- MEM: Multiple erythemata migrantia
- MiQ: Quality standards in microbiological diagnosis of infections
- NAT: Nucleic acid amplification techniques
- NSAR: Nonsteroidal anti-inflammatory drugs
- PCR: Polymerase chain reaction
- p.o. : Per os
- PPI: Proton pump inhibitor
- RCT: Randomised controlled trial
- SNRI: Serotonin norepinephrine reuptake inhibitor
1 Introduction
Lyme borreliosis is the infectious disease most frequently transmitted by ticks in Europe. The Borrelia migrate from the hard-bodied tick Ixodes ricinus into the bite wound during the act of blood feeding. There the Borrelia are either killed off by the (non-specific, innate) immune system, or a localised infection occurs which leads to illness in a small percentage of those infected. Most often the skin becomes inflamed, typically in the form of erythema migrans or, seldom, as borrelial lymphocytoma. In the course of the infection, the Borrelia can disseminate and attack various organs. They primarily affect the skin, joints and nervous system. Acrodermatitis chronica atrophicans can develop as a chronic or late-stage skin infection.
1.1 Target group
This guideline is directed at physicians in private practices and clinics that treat Lyme borreliosis.
1.2 Objectives of this guideline
- Recommendations for confirming a clinical diagnosis
- Recommendations for stage-appropriate laboratory testing: serological detection of IgM and IgG Borrelia antibodies using the 2-step ELISA/immunoblot process; prudent use of procedures for molecular and culture-based diagnostic testing
- Treatment of localised, early-stage infections (erythema migrans, erythema chronicum migrans and Borrelia lymphocytoma)
- Treatment of disseminated early infections (multiple erythemata migrantia, flu-like symptoms)
- Treatment of late-stage infections (acrodermatitis chronica without neurological manifestations)
- Treatment of late-stage infections (acrodermatitis chronica with neurological manifestations)
- Prevention of Lyme borreliosis
- Recommendations for observing tick bites
- Information sheet for patients (Annex 1 in Attachment 1 [Att. 1])
1.3 Participating medical societies and patient organisations
Steering group
Led by:
Prof Dr med. Heidelore Hofmann – coordinator
German Dermatology Society (DDG)
Dr med. Volker Fingerle – coordinator
German Society for Hygiene and Microbiology (DGHM)
Prof Dr med. Sebastian Rauer
German Society of Neurology (DGN) – coordinator
Deputy Dr Stefan Kastenbauer
German Society of Neurology (DGN)
Prof Dr med. Hans-Iko Huppertz
German Society of Paediatrics and Adolescent Medicine (DGKJ) and
German Society of Paediatric Infectiology (DGPI)
Prof Dr med. Klaus-Peter Hunfeld
German United Society of Clinical Chemistry and Laboratory Medicine and
INSTAND e.V.
Prof Dr med. Andreas Krause
German Society for Rheumatology and Clinical Immunology (DGRh)
Prof Dr med. Bernd Salzberger
German Society of Infectious Diseases (DGI)
Consensus group
Prof Dr med. Karl Bechter
The German Association of Psychiatry, Psychotherapy and Psychosomatics (DGPPN)
Dr jur. Astrid Breinlinger
Borreliosis and FSME Association Germany (BFBD)
Ursula Dahlem
Action Alliance Against Tick-Borne Infections Germany (OnLyme-Aktion)
Prof Dr med. Michael H. Freitag
German Society for Occupational and Environmental Medicine (DEGAM)
PD Dr med. Gudrun Gossrau
German Pain Society
Prof Dr med. Gerd Gross
Paul Ehrlich Society for Chemotherapy (PEG)
Prof Dr med. Constanze Hausteiner-Wiehle
German Society of Psychosomatic Medicine and Medical Psychotherapy (DGPM) and the
German College of Psychosomatic Medicine (DKPM)
Prof Dr med. Rainer Müller
German Society of Oto-Rhino-Laryngology, Head and Neck Surgery
Prof Dr med. Mathias Pauschinger
German Society of Cardiology and Cardiovascular Research (DGK)
Prof Dr med. Monika A. Rieger
German Society for Occupational and Environmental Medicine (DGAUM)
Dr med. dent. Herbert Rixecker
German Borreliosis Society (DBG)
Prof Dr rer. nat. Reinhard Wallich
German Society for Immunology (DGI)
Dr Hendrik Wilking
Robert Koch Institute (RKI)
Moderator
Prof Dr med. Ina B. Kopp
AWMF Institute for Medical Knowledge Management
1.4 Methods
This guideline is an updated version of Guideline No. 013-044 “Cutaneous Manifestations of Lyme Borreliosis” Development Stage S1, which was created by an expert committee in 2009.
The guideline was developed in accordance with the methodological requirements of the Association of the Scientific Medical Societies in Germany (AWMF) for the development and further development of diagnosis and treatment guidelines. It is an S2k guideline in accordance with the AWMF’s three-phase concept. The guideline committee was interdisciplinary (IDA) and the appointed mandate holders of the expert medical societies were informed of the planned update on 20 June 2021.
Uniform terms are used in order to standardise the guideline’s recommendations. The following gradation shall apply:
- Strong recommendation: “shall”
- Recommendation: “should”
- Open recommendation: “may be considered”
- Recommendation against an intervention: “should not”
- Strong recommendation against an intervention: “shall not”
2 The microbiology of the pathogen
Five of the six human-pathogenic genospecies from the Borrelia (B.) burgdorferi sensu lato complex have been isolated in Europe: B. afzelii is the most common, followed by B. garinii, B. bavariensis, B. burgdorferi sensu stricto and B. spielmanii [4], [5], [6], [7].
Human pathogenicity has yet to be confirmed for B. valaisiana, B. lusitaniae and B. bissettiae. All of the species that have been confirmed to be human-pathogenic can be found in Europe with the exception of B. mayonii; in the USA only B. burgdorferi sensu stricto and B. mayonii are found, and in Asia all of the species are found with the exception of B. burgdorferi sensu stricto and B. mayonii. The various genospecies of the B. burgdorferi sensu lato complex are genetically very heterogenic [8] and exhibit an organotropism in human infections. Erythema migrans is caused by all 5 genospecies present in Europe, in rare cases possibly also by the relapsing fever Borrelia B. miyamotoi [9]. Acrodermatitis chronica atrophicans is almost exclusively caused by B. afzelii; B. garinii and B. bavariensis are often found in conjunction with neurological manifestations, and B. burgdorferi sensu stricto mainly affects the joints [7], [10]. B. spielmanii has so far only been isolated from erythema migrans [7], [11].
3 Epidemiology
Lyme borreliosis predominantly occurs between the 40th and 60th parallels of the northern hemisphere. Few relevant epidemiological investigations have been conducted in Europe. A population-based study in southern Sweden found an incidence of 69 per 100,000 inhabitants [12]. In a prospective, population-based study of the region around Würzburg over a 12-month period, 313 cases of Lyme borreliosis were identified, which corresponds to an incidence of 111 per 100,000 inhabitants [13]. In terms of early manifestations, localised erythema migrans was diagnosed in 89% of patients and disseminated erythema migrans in a further 3%. Borrelial lymphocytoma was established in 2% of patients, early-stage neuroborreliosis in 3%, and carditis in <1%. With regard to the late forms of the disease, Lyme arthritis occurred in 5% of patients and acrodermatitis chronica atrophicans in 1%. No chronic neuroborreliosis was identified.
Currently there is an obligation to report Lyme borreliosis in nine states in Germany (see Annex 4 in Attachment 1 [Att. 1]) [14]. Epidemiological data obtained through this partial reporting obligation are only based on the clearly diagnosable manifestations, such as erythema migrans, acute neuroborreliosis and acute Lyme arthritis. Thus, it can be assumed that there is a considerable underreporting of cases [14], [15]. A secondary analysis of health insurance data based on the ICD 10 coding A 69.2 (G) found much higher case numbers [16]. A recent study of billing data from statutory health insurances between 2010 and 2019 found that the annual incidence of newly diagnosed Lyme borreliosis cases ranged between 240 per 100,000 insured persons in 2011 and 158 per 100,000 insured persons in 2015 [17].
It can therefore be concluded that the incidence of Lyme borreliosis cannot be conclusively established using the current epidemiological data. Data published to date in Germany indicate that the incidence of Lyme borreliosis is somewhere between 60,000 and 200,000 cases per year, or around 72 to 241 cases per 100,000 inhabitants.
A major, nation-wide seroprevalence study of children (KIGGS) and adults (DEGGS) found that the percentage of Borrelia-specific antibodies in serum rises as population age increases. In 14- to 17-year-olds it is as high as 7%, while in adults, the percentage of Borrelia antibodies is even higher. In the cohort of 70- to 79-year-olds, 24.5% of men and 16.4% of women are seropositive (Figure 1 [Fig. 1]) [18].
Figure 1: Seroprevalence of B. burgdorferi antibodies in Germany – the KIGGS and DEGS studies [18]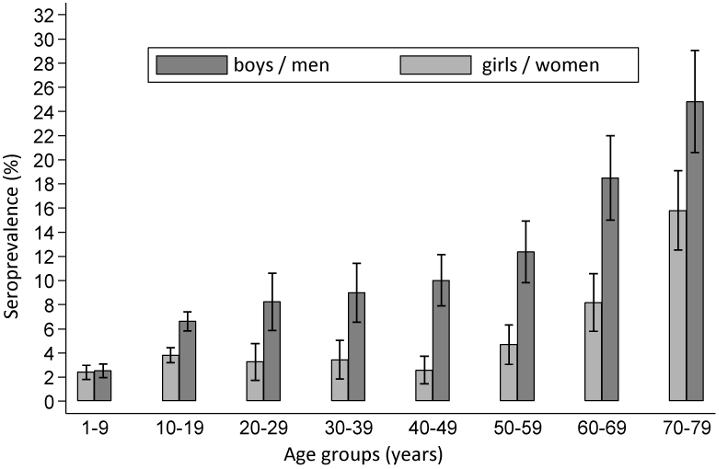
A comparison between two nationally conducted seroprevalence studies (1997–1999 and 2008–2011) shows an annual seroconversion rate of 0.45% (95% CI: 0.37–0.54) of the previously seronegative population. The annual seroreversion rate of the previously seropositive population was 1.47% (95% CI: 1.24–2.17). There was no significant change in seroprevalence between the two study periods [19].
A prospective study of the incidence of Lyme borreliosis in southern Sweden and Finland (2008–2009) revealed that a Borrelia burgdorferi infection occurred in 78 (5%) of the 1,546 people bitten by a tick. Of these, only 45 (3%) experienced a seroconversion and 33 (2%) developed an infection. Erythema migrans was diagnosed in 28 patients, one person had borrelial lymphocytoma, two people had an acute case of neuroborreliosis, and 2 had non-specified symptoms which were diagnosed as Lyme borreliosis [20].
4 Transmission routes
B. burgdorferi is transmitted to birds, mammals and humans from hard-bodied ticks of the I. ricinus/I. persulcatus spp. complex during the blood meal. In Europe it is primarily transmitted from I. ricinus, in northeastern Europe and Asia from I. persulcatus, and in the USA predominantly from I. scapularis.
Often the patient does not recall being bitten by a tick [21]. Ticks suck blood in the course of their cycle of development from larva to nymph to adult tick and before they lay eggs. It is at this time that they can acquire and/or transmit Borrelia. Small rodents – particularly mice – and birds are the primary reservoirs. Birds contribute to the geographical spread of the infected ticks. In Germany, ticks are ubiquitously infected with Borrelia, however percentages can vary greatly from region to region, even between areas very close in proximity (e.g. 4–21%, [22]).
The successful transmission from tick to mammal is the result of a specific, highly complex vector-pathogen interaction. First the Borrelia are activated in the tick’s gut. Then they migrate to the salivary glands where they bind immunosuppressive salivary proteins to their surface [23]. Finally, they are secreted with the saliva into the bite wound where they are – at least partially – protected from the host’s immune system by immunomodulating substances from the tick’s saliva. This is most likely what allows them to reach a sufficiently high infection dose. A similar transmission through blood-sucking insects is close to impossible due to the short blood sucking time (lack of vector competence in insects for B. burgdorferi). Xenobiotic tests reveal that it can take hours for the Borrelia to be transferred depending on the species [24].
See the guideline report for the dissenting opinion of the Borreliosis and FSME Alliance of Germany on transmission from mosquitos [25].
When there is an increased occupational risk of a tick bite, cases of Lyme borreliosis (occupational disease 3102, diseases transmitted from animals to humans) should be reported to the accident insurer by the attending physician or employer as an occupational disease in line with Art. 202 of the Social Security Code VII (see Annex 4 in Attachment 1 [Att. 1]).
5 Pathogenesis
The pathogenesis of the borrelial infection is primarily determined by two factors:
- The pathogen’s evasion strategies [26], [27], [28], [29]
- The quality of the host’s immune response [30], [31], [32], [33], [34], [35], [36], [37]
Moreover, the salivary proteins released by the tick during the blood meal have also been found to have immunosuppressive effects [38], [39], [40], [41], [42], [43], [44], [45], [46].
Host-specific inflammatory reactions in the skin have also been found to influence the course of the infection [47], [48].
Some of the many strategies the Borrelia use to evade the host’s immune system include the ability to mask their cell surface with proteins/inhibitors from the tick or the host, and to modify their phenotype expression of cell surface proteins (outer surface protein: osp) depending on their environment [49], [50], [51], [52].
Several Borrelia species form a resistance to complement-mediated lysis by binding the regulators of the complement cascade (factor H) to their surface [53], [54], [55], [56], [57]. By binding to plasminogens, Borrelia are capable of breaking down collagen, fibronectin and laminin [56], [58], [59], [60], [61] and disseminating in the skin.
The innate immune system recognises the Borrelia mainly by their surface proteins (osp lipoproteins) [62], [63], [64], [65], [66]. This interaction leads to the activation of soluble factors, such as the complement system, as well as to the activation of target cells, like macrophages and dendritic cells, and to the induction of inflammatory cytokines [67], [68], [69], [70]. As the infection progresses, specific immune responses are generated, particularly the activation of T helper cells and B lymphocytes, and the production of Borrelia-specific antibodies [28], [71], [72], [73]. In reservoir hosts, like wild mice, the antibodies that form during an infection are able to prevent disease, however they are unable to eliminate the pathogen. In contrast, the antibodies that form in human patients are often unable to prevent disease. Antibodies against certain Borrelia antigens have, however, also been shown to protect against subsequent infection in humans (see vaccines).
Humans do not acquire permanent immunity following a wild-type infection. As a result, reinfections can occur.
6 Clinical manifestations of Lyme borreliosis
Lyme borreliosis is a multi-organ inflammatory disease. It initially manifests as a localised infection of the skin called erythema migrans. Because of its mild symptoms, this early inflammation of the skin can be overlooked or is not visible. The Borrelia can spread haematogenously. This is clinically recognisable by flu-like symptoms or disseminated erythema on the skin. As the disease progresses, manifestations can appear in other organs, primarily in the nervous system and joints. The disease progresses very differently depending on the individual. Therefore, classifying the disease into stages is not particularly helpful. A distinction between early and late manifestations is preferable since the clinical picture determines both diagnosis and treatment (Table 1 [Tab. 1]). European studies show that Lyme borreliosis manifests as a skin disease in 80–90% of patients and affects other organs in around 10–20% of patients [12], [13], [14], [20].
Table 1: Clinical manifestations of Lyme borreliosis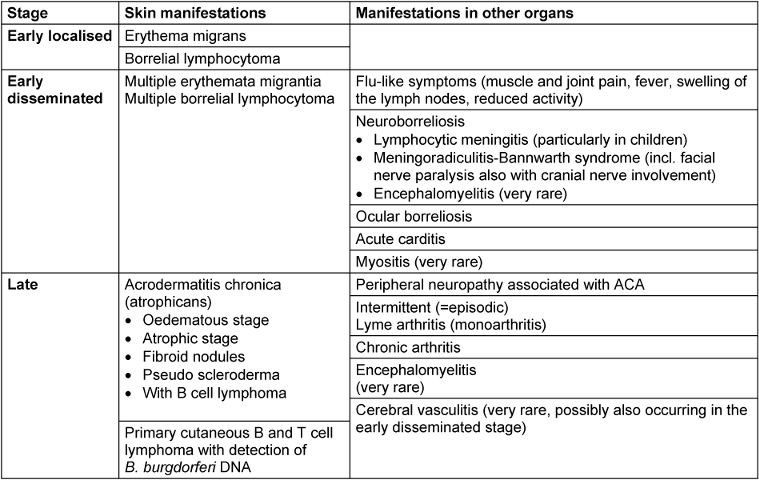
See the guideline report for the dissenting opinion of the Borreliosis and FSME Alliance of Germany on the frequency of erythema migrans [25].
6.1 Early localised cutaneous infections
6.1.1 Erythema migrans
A localised skin infection can occur in the area around the infecting tick bite anywhere from 3 to 30 days following the tick bite [74]. The extent and duration of the inflammatory reaction varies greatly between individuals. A diagnosis of erythema migrans requires the diameter of the erythema to be more than 5 cm (Figure 2A and B [Fig. 2]) [75].
Figure 2: Clinical variations of erythema migrans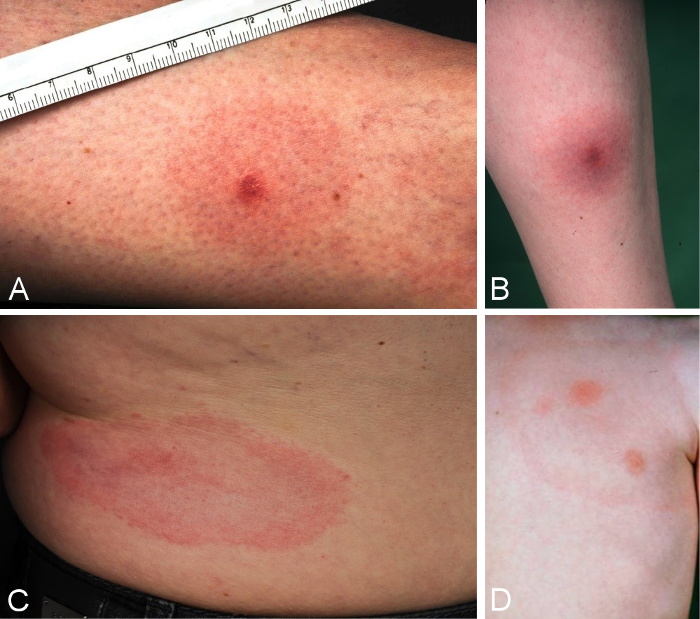
A: initial seronegative erythema migrans one week after tick bite, DD reaction to insect bite; diameter of 4.9 cm, progressive under observation; B: seronegative erythema migrans, increase in IgM antibodies 5 days after start of treatment; C: typical marginated, migrating erythema migrans, D: typical marginated erythema migrans with fresh areas of inflammation inside the ring
A clinically clear presentation of a typical erythema migrans is a marginated erythema – not raised, not overheated – with centrifugal dissemination around the tick bite (Figure 2C and D [Fig. 2]).
Features of a typical solitary erythema migrans
- Time interval between tick bite and onset of the erythema is typically 3 days to several weeks
- Increasing centrifugal spread of the erythema (crescendo reaction)
- Marginated, non-raised erythema that is at least 5 cm in diameter
- Visible tick bite site at the centre of the erythema
(Strong consensus 13/0/0)
6.1.2 Variability of erythema migrans (atypical erythema migrans)
The initial skin infection may be clinically ambiguous. Borrelia have been detected in homogenously red and non-migrating erythema, in patchy and infiltrated erythema (Figure 3B [Fig. 3]), in erysipelas-like flaming red erythema (Figure 3A [Fig. 3]) and in centrally vesicular erythema (Figure 3D [Fig. 3]) [76], [77]. The inflammation can completely disappear in the middle and fade to such as extent that the erythema is only visible around the borders – where the Borrelia are migrating – when heat is applied (Figure 3C [Fig. 3]). The erythema can also be haemorrhagic, particularly on the lower extremities (Figure 3E and F [Fig. 3]). The centre can turn dark purple in colour (Figure 3F [Fig. 3]). The border can be raised or urticarial. The original site of the tick bite is identifiable in the centre as a red papule (Figure 2A and B [Fig. 2]) [76], [78]. In the case of multiple erythema migrantia, erythema also develops at sites other than the site of the bite. Without antibiotic treatment the Borrelia can persist for months or years in the skin and the erythema can slowly spread over the body. Often the red border is the only evidence of the inflammatory reaction to the migrating Borrelia. If the erythema migrans persists for several weeks to months, it is referred to as erythema chronicum migrans [79]: >4 weeks. In most cases (approx. 80%) serological detection of the IgG antibodies (sometimes even the IgM antibodies) is possible [80].
Figure 3: Variability of the erythema migrans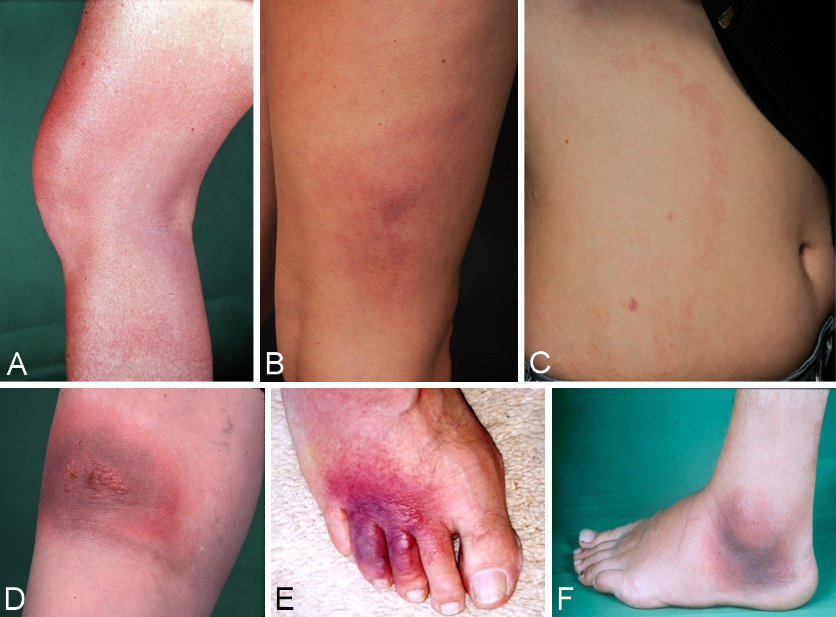
A: flaming red erythema chronicum migrans with radiculitis on the left leg, DD erysipelas; B: blotchy purple erythema chronicum migrans persisting on the upper thigh for 3 months; C: Large light-red arch-shaped erythema chronicum migrans on the abdomen; D: centrally vesicular erythema migrans; E: Haemorrhagic bullous erythema migrans on the foot; F: purple, haemorrhagic, non-migrating erythema chronicum migrans on the outer ankle with joint swelling
The erythema can subside even without antibiotic treatment. Spontaneous healing is possible, however the Borrelia can persist even without a visible inflammatory reaction, and, after a period of latency, this can lead to manifestations in other organs.
Variability of the erythema migrans (atypical erythema migrans)
- Non-migrating
- Not marginated
- Infiltrated instead of macular
- Centrally vesicular
- Haemorrhagic
- Irregular patches
- Only visible when skin is warmed
- No visible tick bite site
(Strong consensus 13/0/0)
Concluding recommendation:
Due to the extraordinary variability of the clinical presentation, atypical erythema migrans is difficult for dermatologically inexperienced physicians to diagnose. Therefore, patients with atypical erythema should be referred to a dermatologist. (Strong consensus 13/0/0)
6.1.3 Borrelial lymphocytoma
In the early stages of the disease, pseudolymphoma (cutaneous lymphoid hyperplasia) (Figure 4B [Fig. 4]) can occur at the site of the tick bite or in the migrating erythema migrans. In most cases it is solitary; in rare cases it is also disseminated. Borrelial lymphocytoma occurs more frequently in children than in adults (in 7% of children and in only 2% of adults with Lyme borreliosis [12]). The preferred sites in children are the earlobes (Figure 4A and C [Fig. 4]), nipples and genitoanal area (Figure 4F [Fig. 4]) [81], [82]. The disease was first described as lymphadenosis cutis benigna by Bäferstedt in 1944. B. burgdorferi s.l. is detectable in the pseudolymphomas [83]. In most cases it is B. afzelii [84]. Histologically, there are mixed B and T lymphocytic infiltrates. However purely B cell infiltrates can also occur, which are difficult to differentiate from low-grade B cell lymphoma (Figure 4D and E [Fig. 4]) [76]. Borrelial lymphocytoma can also occur in the late stages of the disease within the context of acrodermatitis chronic atrophicans [81].
Figure 4: Borrelial lymphocytoma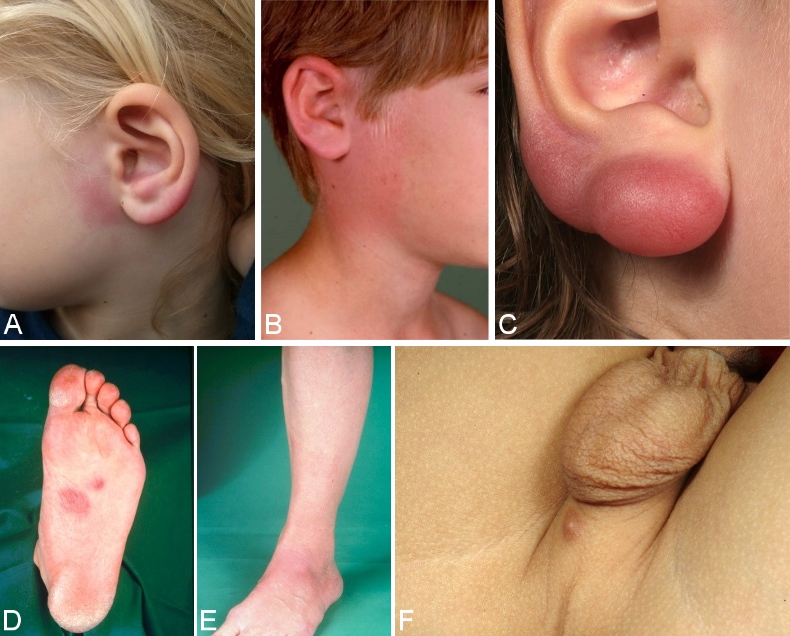
A: Borrelial lymphocytoma preauricular and on the left earlobe; B: Borrelial lymphocytoma on the right auricle near a non-marginated erythema migrans; C: pronounced knotty borrelial lymphocytoma on the earlobe; D, E: Borrelial lymphocytoma on the sole of the foot with erythema migrans on the lower leg, initially misdiagnosed histologically as a low-grade B cell lymphoma; F: small perineal borrelial lymphocytoma
In the case of borrelial lymphocytoma, there is a substantial increase in the number of IgG antibodies in the serum regardless of the duration of the infection [85], [86]. In rare cases, multiple borrelial lymphocytomas can occur in the early disseminated stage or even in the late stages of the disease. Here accurate, histological, immune-histochemical and molecular-genetic clarification is required so that a differential diagnosis can be made from malignant cutaneous lymphomas.
Important features of a borrelial lymphocytoma
- Pseudolymphoma, mostly solitary, more frequent in children
- Localised, primarily on the earlobes, nipples or in the genital area
- Purple subcutaneous nodules or plaque
- Histologically, mostly mixed B and T lymphocytic infiltrates
(Strong consensus: 13/0/0)
6.2 Early disseminated cutaneous manifestations
Some patients experience haematogenous dissemination in the early stages of the disease, which is clinically identifiable by flu-like symptoms such as a slight fever, arthralgia, myalgia, headache, lymphadenopathy and multiple erythemata migrantia. This stage is very difficult to diagnose if no erythemas are visible or if they cannot be identified because they have an atypical morphology.
Multiple erythemata migrantia (MEM)
The haematogenous dissemination of the Borrelia in the skin is identifiable by the many sharply defined, symptomless, oval erythemas of varying sizes, i.e., multiple erythemata migrantia (Figure 5B and C [Fig. 5]) [77], [87], [88]. Children often experience symmetrical erythema on their face, similar to fifth disease (Figure 5A [Fig. 5]) [76], [77]. MEM can be associated with systemic symptoms and acute neurological symptoms [89]. The histological picture is initially atypical. The typical perivascular plasma cell infiltrates can only be found in the advanced stage of the disease. There is usually a highly elevated number of IgM antibodies in the serum, or the antibodies increase rapidly once treatment begins. There is usually an elevated number of IgG antibodies. Borrelia taken from skin lesions, and, in rare cases, blood can be cultivated, or their DNA can be detected by PCR [88], [90].
Figure 5: Multiple erythemata migrantia (MEM)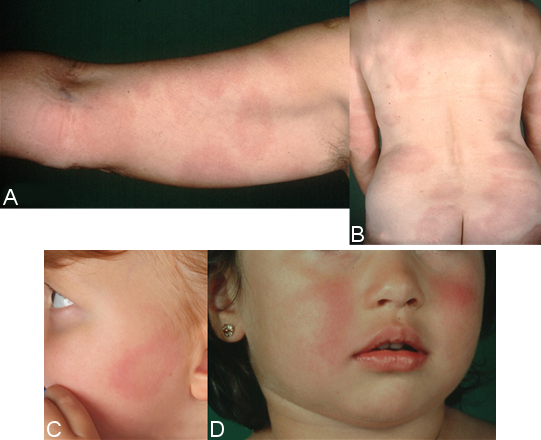
A, B: pronounced erythemata migrantia on the right arm, approx. 40 more on the torso and lower extremities; C: oval erythema on the left cheek, several similar erythema on the torso and thigh during early disseminated borreliosis; D: symmetrical redness on the cheeks in a 5-year-old girl with multiple erythemata migrantia on her torso and extremities, accompanied by flu-like symptoms
Important features of multiple erythemata migrantia
- Symptomless, disseminated round or ovular redness on the skin (strong consensus: 13/0/0)
- No epidermal changes
- Ring-shaped or homogenous
- In children, often symmetrical erythema on the face (similar to fifth disease)
- Persisting days or weeks
- Recurring at the same sites
- Possibly associated with systemic or acute neurological symptoms
(Strong consensus: 13/0/0)
6.3 Late cutaneous manifestations
Acrodermatitis chronica atrophicans (ACA)
The disease can manifest in various organs after varying periods of time – from months to years – depending on the individual. Chronic skin infections mostly occur in older patients and more frequently in women [3], [91].
A large retrospective descriptive cohort study conducted over 28 years (1991–2018) of 693 Slovenian patients who presented with ACA has now been published for the first time. Clinical and microbiological findings were anal-ysed. A diagnosis was made after a mean time of 12 months following the onset of changes to the skin. The lower extremities were affected in 70% of cases. In 55% of patients, the ACA was localised to one extremity, in 31.3% to 2, in 5.6% to 3 and in 7.8% to all 4 extremities. Bilateral involvement was observed in 42.1% of cases. Fibroid nodules were also reported in 2.2% of patients, concomitant arthritis in 2.6% and in 20.8% of patients, the ACA was associated with peripheral neuropathy. The neuropathic symptoms only occurred in the extremity affected by the ACA [3].
Isolated cases have also been reported in children [86], [92], [93], [94], [95].
Oedematous-infiltrative stage of ACA
Acrodermatitis initially manifests as pink reticular, then increasingly purple, oedematously infiltrated cushion-like erythema, usually on the extremities. The skin is excessively warm, however there is initially no pain apart from a feeling of heaviness. This is the oedematous-infiltrative stage of acrodermatitis chronica (Figure 6A and B [Fig. 6]). These purple infiltrates can also appear on the face and be mistaken for lupus erythematosus or cutaneous malignant lymphoma [76].
Figure 6: Late cutaneous manifestations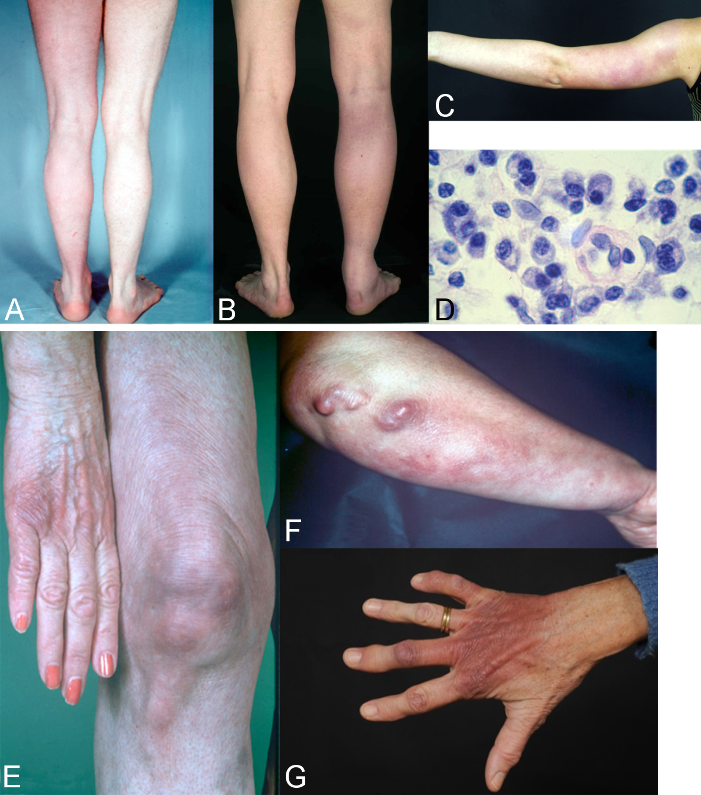
A–D: Oedematous-infiltrative stage of acrodermatitis chronica. A: Acrodermatitis chronica. Homogenous reddening of the left leg without atrophy, persisting for one year; B Acrodermatitis chronica in the oedematous-infiltrative stage. Pronounced swelling and purple discolouration of the right leg with nodular enlargement of the Achilles tendon and swelling of the ankle joint; C: Acrodermatitis in the oedematous-infiltrative stage. Blotchy purple confluent erythema on the left arm of a 15-year-old girl; D: typical perivascular plasmacellular infiltrate in acrodermatitis chronica
E–G: Atrophic stage of ACA. E: ACA Purple discolouration and atrophy on the back of the right hand and little finger, and purple blotches, bands and infiltrates dorsally on the right knee; F: ACA with ulnar stripes and purple blotches on the right underarm and pronounced purple fibroid nodules below the elbow; G: ACA dark red to purple discolouration and atrophy of the right hand dorsally (so-called “baked apple skin”) with swelling of the finger joints
Atrophic stage of ACA
In the course of the disease there is increasing atrophy of all skin layers and skin appendages. Occasionally coarse, juxta-articular fibroid nodules and band-shape stripes appear (Figure 6F [Fig. 6]), e.g. rare but typical inflammatory ulnar stripes and swelling in the heel and Achilles tendon, or in other joints around the ACA, even in the oedematous-infiltrative stage (Figure 6B [Fig. 6]). As the disease progresses, circumscribed fibrosis or pseudoscleroderma develops near the ACA, which can be mistaken for circumscribed scleroderma. Arthritis, arthralgia and myalgia in the affected extremities are frequently associated with ACA [91].
A peripheral neuropathy occurs in 40–60% of patients in connection with ACA, which is characterized by a feeling of numbness, a tingling sensation, burning and an increased sensitivity to pain (allodynia) [96], [97], [98]. Without antibiotic treatment, Borrelia can live for years in the skin and in the fibroid nodules [99].
As the disease progresses, all of the affected skin atrophies and there is a loss of body hair as well as connective and fatty tissue (Figure 6E and G [Fig. 6]).
When the changes to the ACA-affected skin are symmetrical, they are difficult to differentiate clinically from age-related skin atrophy, acrocyanosis and chronic venous insufficiency. Histologically, acrodermatitis chronica atrophicans is characterised by a pronounced perivascular plasma cell-rich inflammatory infiltrate throughout all layers of the skin (Figure 6D [Fig. 6]) and, in the late stages, by an increasing atrophy of the epidermis, connective tissues and fatty tissues [100].
An ACA diagnosis is based on a typical clinical presentation, a typical histology and, as a rule, a strong positive detection of Borrelia IgG antibodies in serum [3], [91]. In ambiguous cases, particularly in the case of borderline antibody concentrations, a diagnosis must be confirmed by histological analysis and DNA detection, or by cultivating Borrelia from the skin.
Important clinical features of acrodermatitis chronica atrophicans (ACA)
- Initial oedematous-infiltrative stage (plasmacellular dermatitis), reddish discolouration of the skin, usually on one extremity
- As the disease progresses, transition to the atrophic stage: purple to loam-brown discolouration of the skin, skin atrophy, loss of body hair, loss of connective and fatty tissues, protrusion of blood vessels, juxta-articular fibroid nodules and joint involvement
- Association with a peripheral neuropathy in around 50% of cases
- Older women more likely to be affected
(Strong consensus: 13/0/0)
6.4 Manifestations in the nervous system and joints associated with cutaneous borreliosis
Early borreliosis with erythema migrans can be accompanied by early neuroborreliosis. Arnez et al. found pleocytosis in the cerebrospinal fluid in 26% of the 214 children with multilocular erythemata migrantia that they studied. Of these, 11% had clinically symptomatic lymphocytic meningitis [101]. Bannwarth Syndrome (radiculoneuritis) with characteristic nocturnal pain can also occur – in rare cases with paresis of the cranial or peripheral nerves.
A peripheral neuropathy of the affected extremity occurs in 50% of patients with ACA [97].
Rheumatological symptoms, especially myalgia and arthralgia, can occur relatively early on in the disease alongside erythema migrans. Attention should be paid to cardiac symptoms with dysrhythmia (typically AV block of varying degrees), which can occur in conjunction with or following a case of erythema migrans.
Frequently, the joint adjacent to the erythema migrans is affected. This typically manifests as acute intermittent arthritis of the large joints with voluminous, at times painful joint swelling, usually in the form of mono- or oligoarthritis. Knee joints are affected in 85% of patients. The, often, extensive knee joint effusions lead unusually frequently and early on to the development of popliteal cysts (Baker’s cysts). Ankle and elbow joints are less frequently affected and no involvement of the finger joints, especially in the form of polyarthritis, has been observed. Lyme arthritis is usually episodic, i.e. with recurring bouts of inflammation that alternates between periods of light to no symptoms.
6.5 Primary cutaneous lymphomas with a B. burgdorferi infection
A recent meta-analysis on the association between B. burgdorferi and primary cutaneous lymphomas found there is a significant association in areas where Borrelia are endemic. Here no differences were detectable between the different lymphoma entities (both B-cell and T-cell lymphomas). The authors recommend molecular testing for B. burgdorferi and, if necessary, antibiotic treatment for patients from endemic areas that have primary cutaneous lymphoma. However, further studies are deemed necessary to confirm this finding [102].
6.6 Differential diagnoses for cutaneous Lyme borreliosis
The most common differential diagnoses for cutaneous Lyme borreliosis are listed in Table 2 [Tab. 2].
Table 2: Clinical differential diagnoses of cutaneous Lyme borreliosis 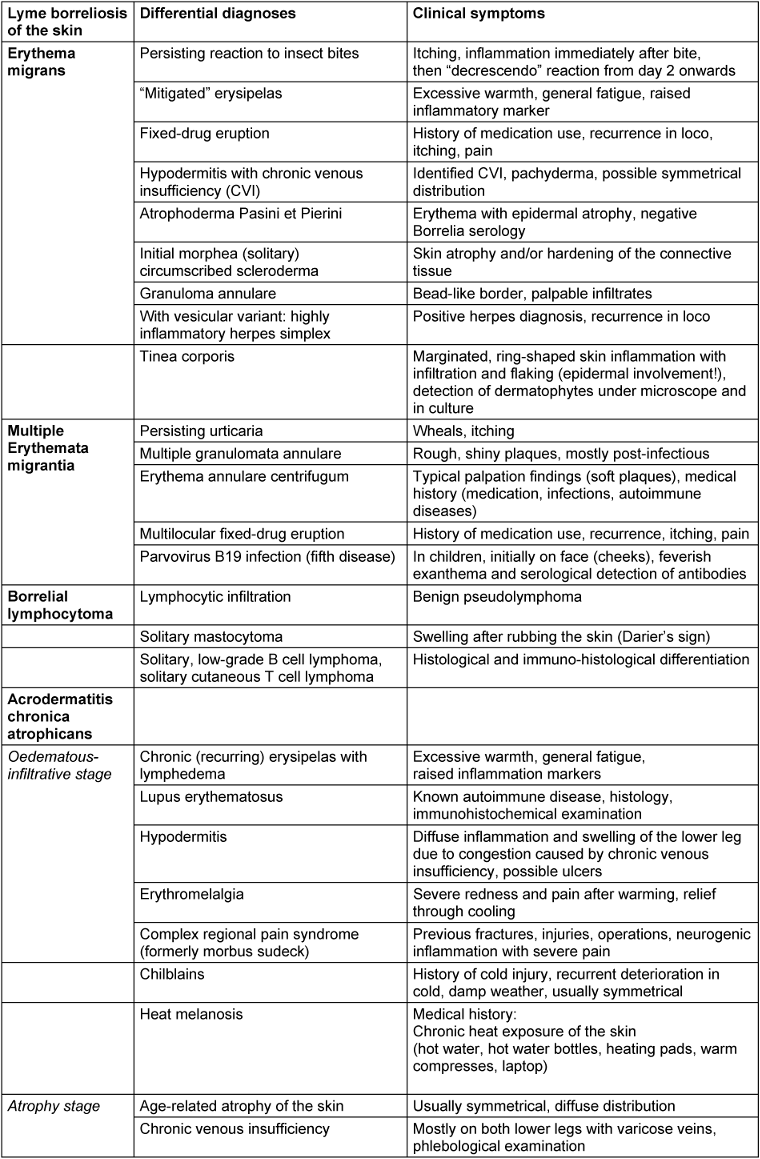
The multitude of differential diagnoses are evidence that, with the exception of typical erythema migrans, most cutaneous manifestations of Lyme borreliosis require careful dermatological testing. In particular, a poor response to antibiotic treatment should not be uncritically interpreted as persistent borreliosis that is then treated for months with antibiotics. In such cases, the initial diagnosis must be critically questioned and a differential diagnosis be made. This avoids unnecessary, potentially harmful antibiotic treatment or a delay in treatment for other diseases, or it confirms the initial diagnosis.
It is therefore recommended that patients whose ambiguous skin afflictions persist after treatment, be referred to dermatologists or to paediatricians with dermatological experience.
Recommendation
Skin inflammations that were diagnosed as Lyme borreliosis and which have not healed after lege artis antibiotic treatment shall be referred to a dermatologist. (Strong consensus 13/0/0)
7 Diagnostic testing
7.1 Indirect pathogen detection (serodiagnosis, antibody detection)
Due to the complex characteristics of the pathogen, indirect pathogen detection using serological methods continues to play a key role in the diagnosis of Lyme borreliosis in routine laboratory medical care. In accordance with the methods and standards required in Germany, antibodies are detected in serum using a step-by-step diagnostic approach made up of a standardised screening test (immunoassay: ELISA, CLIA etc.) and a confirmation test (immunoblot). This ensures that the diagnostic procedure achieves the highest possible sensitivity and specificity (Table 3 [Tab. 3]).
Table 3: Step-by-step approach to serology testing (as per MIQ 12 and DIN 58969-44 2005-07) 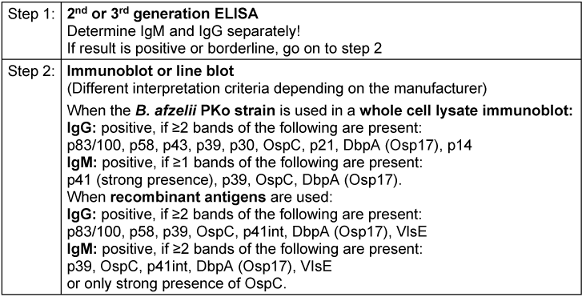
In Europe, serology tests for Borrelia are not subject to any form of mandatory, extensive or independent clinical evaluation as part of the approval process. This means that there is a range of commercially available test formats. In addition to various types of immunoassays, there are also a variety of test antigen preparations that use native and recombinant antigen combinations with, at times, varying performance data [16], [103], [104]. Even though the principles of testing procedures and the interpretation of serology test results are laid down in binding standards in Germany (DIN 2005), it should be noted that the variability and insufficient standardisation of commercial test systems means that the interpretation of test results and, in particular the evaluation criteria for immunoblot tests, vary among manufacturers and must be carried out according to the respective manufacturer’s specifications. Meta-analyses conducted as part of external quality controls highlight the ongoing issue of insufficient standardisation of testing and comparability of results [16], [105]. Thus, attending physicians should be aware of the credentials of their diagnostic laboratory as well as the diagnostic assays and test specifications that it uses and, where possible, have the laboratory that performed the initial test perform the follow-up test or, preferably, use the same test while applying a parallel approach with the pre-serum.
Progression of the immune response and the interpretation of findings
In the course of a natural infection, specific IgM antibodies are usually detectable 3–6 weeks after the onset of illness; IgG antibody titres peak more slowly (after weeks to months).
It should be noted that seroconversion may not occur even after timely and successful treatment of early manifestations. Furthermore, when IgM antibodies are detected, the immune response may not progress normally in the sense of a conversion from IgM to IgG. In contrast to immune responses to other infectious bacterial diseases, the antibody response with Lyme borreliosis often regresses very slowly after both a latent and resolved infection as well as after successful treatment. As a result, IgM reactivities or specific IgG values may remain detectable for months, even years, after the patient has successfully recovered from the infection. Weakly positive borrelial-specific antibody levels are often a sign of a previous infection in the sense that antibodies are left over from a past infection (serological scar) [19], [103], [104], [106]. Nevertheless, a reinfection cannot be ruled out based on these laboratory results. Such results were detected in 20% of people belonging to frequently exposed population groups, e.g. forestry workers, who were examined as part of health screenings without any current or past symptoms of disease [107], [108]. Possible co-incidences of these types of titres, with persisting antibodies from previous infections and unspecified findings, are also possible among the normal population [18], [109] and can be responsible for erroneous interpretations and diagnoses. It is therefore advisable to always complete the ‘Symptoms’ section on the laboratory order. This supports the laboratory in better interpreting the results.
In immunocompetent patients, the isolated positive detection of IgM antibodies effectively rules out a late manifestation of Lyme borreliosis.
The use of very sensitive early-phase antigens, such as VlsE, in diagnostic testing means that a specific IgG response can be detected very early on in the course of the infection. As a result, specific IgM antibody results are playing an increasingly limited role in the diagnosis of Lyme borreliosis, especially as IgM detection has a poorer overall specificity than IgG detection [110], [111]. However, positive IgG levels can also persist, sometimes in high concentrations, over a long period of time [19], so that no conclusions can be drawn regarding the activity of Lyme borreliosis or even the need for treatment in the absence of a traditional activity marker, without additional clinical information and only on the basis of positive serological findings. At the same time, a statement can only be made about the significance of changes in the findings if comparison tests are carried out on serum samples that were taken at different times, ideally using a parallel approach with the pre-serum, but, in any case, using the same test [103], [112].
An immunoblot analysis is generally used within the framework of a step-by-step diagnostic approach to confirm the results of the screening test as well as to classify the immune response into an early or late phase based on the band pattern, especially in the case of the IgG immunoblot. Thus, a narrow spectrum of detected bands with antibodies against early-phase antigens (e.g. VlsE, OspC, p41) typically corresponds to an early manifestation (e.g. erythema migrans, facial paresis) or a brief latent infection; it does not correspond to an infection that has persisted for some time [103], [106], [112], [113], [114]. In contrast, a wide band spectrum, including reactions to late-phase antigens (e.g. p100, p17/p18), corresponds well to a late manifestation (e.g. arthritis, acrodermatitis) [106], [113], [114] as well as to the asymptomatic persistence of antibodies (serological scar). It primarily does not, however, correspond to an early manifestation or a short duration of the disease. Reinfections are difficult to detect based solely on serology test results and without additional clinical information. They are only detectable by a clear increase in IgG levels or significant changes in the immunoblot band pattern in serum samples examined in parallel.
The immunoblot must be interpreted by an experienced laboratory technician, making it time-consuming and expensive, and results can vary depending on the examiner. Therefore, it has been suggested that the immunoblot be replaced by another ELISA – e.g. with C6 peptide [115], [116]. This simplifies the serology test. As the result depends on the quality of the primary ELISA, both parts of the two-step test procedure can be carried out consecutively or in parallel [115], [117]. In the USA, several ELISAs have already been authorised by the FDA to be used in such a procedure. However, this can naturally lead to a loss in specificity. This procedure also makes it impossible to assess the development of the immune response – in the sense of a widening of the band pattern – which can be important for the serological assessment of late or chronic forms of the disease. Further investigations need to be conducted to establish whether this approach is also suitable for Europe, and if so to what extent.
One major premise of serological testing for Lyme borreliosis is that the requesting physician is aware that these types of tests should only be ordered if there is reasonable clinical suspicion, i.e. the patient’s clinical findings and subjective complaints must always be taken into account. Only when there is sufficiently high pre-test probability (prevalence of Lyme borreliosis in the patient cohort being investigated >20%) can it be assumed that there is a sufficiently utilisable positive predictive value of a positive test result [118]. If the test is only ordered to rule out Lyme borreliosis when there are unspecified or non-typical disease symptoms, the positive predictive value of the laboratory test drops to almost zero with respect to the possible confirmation of Lyme borreliosis. However, due to the relatively low overall prevalence and incidence of Lyme borreliosis among the general population, a negative test result has an excellent negative predictive value for ruling out the disease in immunocompetent patients with persisting symptoms.
Recommendations
- Serology testing shall only be ordered when there is sufficient clinical suspicion. (Strong consensus: 13/0/0)
- Testing shall be performed using a step-by-step diagnostic approach (screening and confirmation test). (Consensus: 11/2/0)
- A positive antibody test is not proof that Lyme borreliosis is clinically present. (Strong consensus: 13/0/0)
- A negative antibody test largely rules out Lyme borreliosis in immune-healthy patients with a protracted length of illness. (Consensus: 11/2/0)
- An isolated positive IgM test does not support a late manifestation of Lyme borreliosis. (Consensus: 11/2/0)
See the guideline report for the dissenting opinion of the Borreliosis and FSME Alliance of Germany on assessing serology testing [25].
7.2 Direct pathogen detection
For Lyme borreliosis, the relevant quality standards for microbiological analysis apply to direct pathogen detection using culture and PCR/NAT [106], [119].
7.2.1 Culture
In the past, direct detection by culture using the modified Barbour-Stoenner-Kelly medium was the gold standard and provided clear proof of an infection with B. burgdorferi [120], [121]. Now, however, it is rarely used. Direct detection of skin manifestations by culture are often successful. Detection by culture can be done to a limited degree from cerebrospinal fluid, and very rarely from synovial fluid, synovial biopsies and blood. In individual cases, the detection of B. burgdorferi has also been achieved in other tissue samples, e.g. heart muscle and iris [122], [123]. Cultivation from patient samples using suitable media is material- and labour-intensive and commonly takes more than two weeks. European studies have found the sensitivity of these methods to be between 40% and 90% for erythema migrans and between 20% and 60% for ACA [124], [125], [126], [127]. Overview in: [128]. Because of the invasiveness of the sample taking, direct detection by culture should therefore be based on a clear indication and be explicitly restricted to specially designated reference laboratories, such as the National Reference Centre for Borrelia at the Bavarian Health and Food Safety Authority in Oberschleissheim. This is particularly important as further molecular confirmation tests are required in the event of a positive result.
Recommendations for direct detection by culture
- Direct detection by culture should only be performed if the differential diagnosis is inconclusive. (Strong consensus: 13/0/0)
- The cultivation of Borrelia burgdorferi sensu lato should remain restricted to specialist laboratories. (Strong consensus: 13/0/0)
- Positive culture results are to be confirmed using suitable molecular methods. (Strong consensus: 13/0/0)
7.2.2 Direct detection using molecular methods
The detection methods currently being used in Lyme borreliosis testing are regarded as having a low level of standardisation [129]. This applies to DNA isolation from suitable clinical materials, as well as to reaction conditions and the selection of primers. In principle, the detection of Borrelia from a skin biopsy using nucleic acid amplification techniques (NAT, usually PCR) is very reliable and, in the case of early manifestations, is more sensitive than serological antibody detection. The diagnostic sensitivity of NAT for detecting erythema migrans and acrodermatitis chronica atrophicans from biopsies is around 70% [127], [130], [131], [132], [133]. However, molecular confirmation tests (probe hybridisation, sequencing of the amplificate) must confirm the specificity of the positive results and these findings must be reported.
After treatment, Borrelia DNA can still be detected for weeks – or even months – in the affected patch of skin before conclusions can be drawn as to whether the therapy has failed [134], [135], [136]. The molecular detection of pathogens without the simultaneous presence of typical disease manifestations is not clinically relevant. Direct molecular detection in urine samples is not currently recommended as the diagnostic sensitivity and specificity are unclear [133], [137], [138]. Direct detection by PCR should therefore be based on a clear indication (e.g. skin manifestations with inconclusive differential diagnosis) due to the invasiveness of the sample collection and lack of standardisation. Thus, it should be explicitly restricted to designated specialist and reference laboratories, such as the National Reference Centre for Borrelia at the Bavarian Health and Food Safety Authority in Oberschleissheim. This is particularly important since molecular confirmation tests are required in the event of a positive result.
Recommendations for molecular detection
- Direct molecular detection (PCR) is not a screening test if Lyme borreliosis is suspected. (Strong consensus: 13/0/0)
- A negative PCR test does not rule out Lyme borreliosis. (Strong consensus: 13/0/0)
- A positive PCR test shall be confirmed by further molecular testing methods and the detected genospecies shall be reported in the findings. (Strong consensus: 13/0/0)
- A positive PCR test following treatment with antibiotics in accordance with the guidelines or without a clinically typical manifestation is not an indication for (renewed) treatment with antibiotics. (Consensus: 11/2/0)
- Direct molecular detection should only be used for ambiguous skin manifestations and be conducted in specially designated microbiology labs. (Strong consensus: 13/0/0)
7.3 Diagnostic testing of clinical skin manifestations
7.3.1 Erythema migrans (typical)
If a clinically typical erythema migrans is present (see section on clinical manifestations), no further laboratory testing should be carried out and antibiotic treatment should start immediately (Figure 7 [Fig. 7]).
Figure 7: Algorithm for diagnosing a solitary or multilocular erythema migrans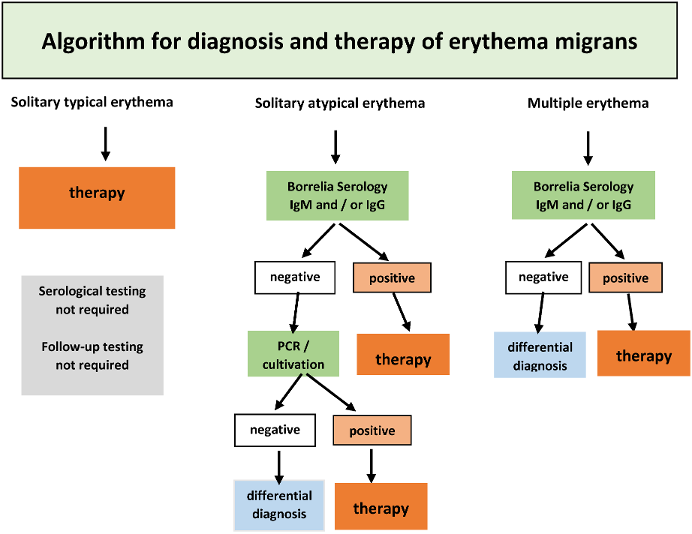
Recommendations
- If a typical erythema migrans is present (see section on clinical manifestations), no further laboratory testing (serological, cultural or molecular) is required. (Strong consensus: 13/0/0)
- If a typical erythema migrans is present, antibiotic treatment shall begin immediately. (Strong consensus: 13/0/0)
7.3.2 Erythema migrans (atypical)
If an atypical erythema migrans is suspected, a serology test shall be carried out in every case (Table 4 [Tab. 4]). If the findings remain ambiguous, pathogen detection should be done using PCR, if necessary, also by culture (Figure 7 [Fig. 7]). A skin biopsy should be taken near the inflamed border. After informing the patient and obtaining written consent, the selected area of the skin is numbed using local anaesthesia. After thorough disinfection of the skin, a 4 mm punch is used to remove the skin. This is then placed in a sterile vessel with a 0.9% saline solution. Direct inoculation in the culture medium only makes sense if the sample can be processed in a laboratory within a few hours, as otherwise, fast growing skin bacteria can make it difficult to cultivate the Borrelia.
Table 4: Interpretation of the constellations of the serological results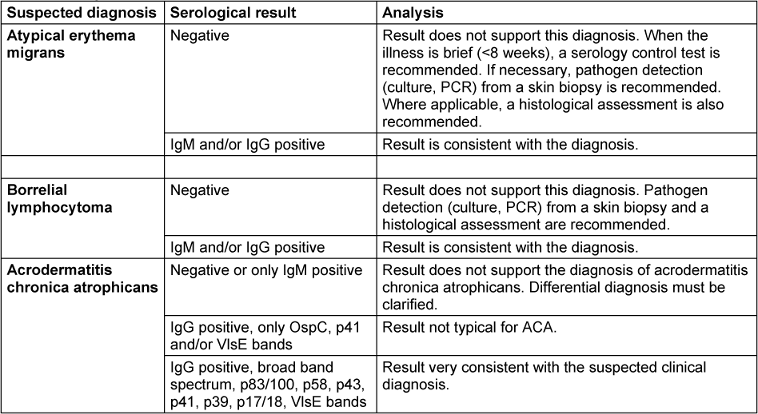
A histological analysis is rarely able to indicate if it is erythema migrans; it can, however, be useful in making a differential diagnosis.
Recommendations
- In the case of an atypical clinical appearance of erythema migrans, suspicion shall be clarified by a serology test. (Consensus: 11/2/0)
- If the serology test is negative and clinical suspicion remains, direct molecular detection or direct detection by culture from biopsy material shall be used for clarification. (Strong consensus: 13/0/0)
7.3.3 Multiple erythemata migrantia (MEM)
If multiple erythemata migrantia, also known as multilocular erythema migrans (MEM) is suspected, serological antibody detection and pathogen detection by PCR and culture from a skin biopsy can be used. A serology test should always be carried out. If the findings remain ambiguous, a PCR test should be used to detect the pathogen, if necessary, also by culture (Figure 7 [Fig. 7]) (see 3.2.1). In the case of MEM, special attention should be paid to clinical signs of extracutaneous symptoms (see Table 3 [Tab. 3] in the section on clinical manifestations).
Recommendations
- A serology test should be conducted if MEM is suspected.
- If the serology test is negative and clinical suspicion of MEM persists, further clarification is needed by means of direct molecular detection or direct detection by culture from biopsy material. (Strong consensus: 13/0/0)
7.3.4 Borrelial lymphocytoma
Diagnoses must be confirmed by serological antibody detection (Table 4 [Tab. 4]), whereby in most cases antibodies against B. burgdorferi can be detected [47], [76], [139]. When findings remain inconclusive, patients shall be referred to a dermatologist so that the pathogen can be detected by PCR or, if necessary, by culture. Two skin biopsies should be taken from the altered patch of skin (see 3.2.2) – one in a 4% formalin solution for the histological assessment, and one in a sterile, physiological saline solution for the culture and PCR test.
B. burgdorferi can be detected by PCR and culture. Although there is limited data on the sensitivity of the methods with respect to borrelia lymphocytoma, PCR detection has a success rate of 70% [81], [139].
Recommendations
- If there is an unambiguous clinical presentation of borrelial lymphocytoma and a positive serology, further microbiological tests are not required. (Strong consensus: 13/0/0)
- If there is an unambiguous clinical presentation of borrelial lymphocytoma, antibiotic treatment is to begin immediately. (Strong consensus: 13/0/0)
- If the clinical presentation is not unambiguous and the serology is negative, further tests (primarily histology, molecular biology, possibly culture) are to be conducted to determine the differential diagnosis. (Strong consensus: 13/0/0)
7.3.5 Acrodermatitis chronica atrophicans
If there is a clinical suspicion of ACA, a Borrelia serology test should be carried out first (Table 4 [Tab. 4]). High antibody levels in the IgG screening test, combined with a broad spectrum of borrelial-specific bands in the IgG blot or similar tests (see section 4.1.1), are indications of ACA. A negative IgG serology rules out, with high certainty, ACA in immunocompetent patients.
In a large cohort of 693 Slovenian patients diagnosed with ACA within a period of 28 years, all patients were found to have highly elevated levels of IgG antibodies, and 30% also had elevated levels of IgM antibodies [3]. The detection of plasma cells in a skin biopsy is an additional indicator that helps to confirm the diagnosis.
In ambiguous cases the patient should be referred to a dermatologist for differential diagnosis. If uncertainty remains, two skin biopsies should be taken from the altered patch of skin (see 3.2.2) – one in a 4% formalin solution for the histological assessment, and one in a physiological saline solution for the culture and/or PCR test. In the case of ACA, B. burgdorferi DNA can be detected in around 70% of patients.
Recommendations
- If there is clinical suspicion of ACA, the diagnosis shall be confirmed by a serology test. (Strong consensus: 13/0/0)
- High IgG antibody levels in the screening test in combination with a broad band pattern in the IgG immunoblot support the suspected clinical diagnosis. (Strong consensus: 13/0/0)
- A negative Borrelia serology rules out ACA with a high degree of certainty in immunocompetent patients. (Consensus: 12/0/1)
- Histological confirmation of the diagnosis should be carried out. (Strong consensus: 13/0/0)
- When the clinical presentation is ambiguous, further diagnostic clarification should be carried out with a biopsy and subsequent histological assessment. If the findings are unclear, direct detection by culture and molecular methods is recommended. (Strong consensus: 13/0/0)
7.3.6 Ambiguous dermatological pathologies for a suspected case of Lyme borreliosis
Recommendations
- If a cutaneous manifestation of Lyme borreliosis is suspected and the clinical presentation is ambiguous, a skin biopsy with a fine-tissue (histology) assessment should be carried out together with direct pathogen detection by culture and molecular methods. (Strong consensus: 13/0/0)
- If the clinical presentation is ambiguous and the serological and histological assessments are negative, an enhanced interdisciplinary diagnosis should be performed to clarify the differential diagnosis. (Strong consensus: 13/0/0)
7.4 Non-recommended diagnostic approaches
In addition to the traditional diagnostic methods listed above, which are used for a suspected case of Lyme borreliosis, the literature describes a range of other diagnostic methods, some of which have not been fully evaluated. This includes the immunohistochemical detection of B. burgdorferi in biopsies, antigens in blood and urine, a CD57-positive/CD3-negative lymphocyte subpopulation, dark-field microscopy, ‘xenodiagnosis’ (hard-bodied tick larvae feed on the blood of patients with suspected Lyme borreliosis; the larvae are then analysed for Borrelia) as well as functional tests to test cellular immunity (lymphocyte transformation tests (LTT), cytokine detection). Currently there is a lack of scientific studies that prove there is a diagnostic benefit. In particular, LTTs should not be used as the tests that are currently available have a poor specificity. A current systematic review and an independent study on cellular testing show that no reliable evidence can be derived from the scientific literature to justify the use of these tests in routine diagnostic testing [140], [141].
The following approaches should not be used to diagnose cutaneous manifestations of Lyme borreliosis:
- The immunohistochemical detection of Borrelia in tissue (strong consensus: 13/0/0)
- PCR in serum and urine (strong consensus: 13/0/0)
- Lymphocyte transformation tests (LTT), enzyme-linked immunospot assay (ELISPOT) and/or the detection of specific cytokines (consensus: 12/1/0)
- The detection of Borrelia in feeding ticks (consensus: 12/0/1)
- The detection of Borrelia antigens in patient samples (strong consensus: 13/0/0)
- Direct microscopy to detect Borrelia in patient samples (strong consensus: 13/0/0)
- The detection of circulating immune complexes (strong consensus: 13/0/0)
- The detection of so-called L-forms or spheroblasts (strong consensus: 13/0/0)
- Rapid antibody tests (point-of-care tests (POCT)) due to lack of sensitivity (18–32%) (strong consensus: 13/0/0)
- “Visual Contrast Sensitivity Test” (VCS test or grey shade test) (strong consensus: 13/0/0
7.5 Quality control and quality assurance
According to the current guideline of the German Medical Association, diagnostic laboratories must participate twice a year in external quality assessment (EQA) schemes for serological infectious disease testing. This applies to serological antibody detection and to direct molecular detection of Borrelia when Lyme borreliosis is suspected. INSTAND e.V. has been carrying out such EQA schemes for many years. The results of these EQA schemes reveal an extensive heterogeneity in the test systems currently on the market. The pass rates for conventional serological and molecular test systems taken from meta-analyses indicate that, even though the immunoassays and molecular tests have good analytical pass rates, a clinical diagnostic interpretation of result constellations often proves difficult and can complicate medical care in everyday clinical practice [16], [103]. Thus, when Lyme borreliosis is suspected, diagnostic infectious disease testing is to be conducted in laboratories that meet the diagnostic standards as per the guidelines of the medical societies and the guideline of the German Medical Association. These laboratories must successfully participate in external quality assessment schemes (EQAs) on a regular basis. Physicians treating patients with Lyme borreliosis should enquire about and ensure that these conditions are met by the laboratories charged with carrying out their diagnostic testing. The laboratories specialising in Lyme borreliosis testing and the National Reference Centre for Borrelia at the Bavarian Health and Food Safety Authority in Oberschleissheim should be consulted in the event that there are questionable result constellations or implausible test results.
Recommendation
- Attending physicians shall be aware of whether their testing laboratory complies with the relevant diagnostic standards and has the necessary competencies. They shall also be aware of the extent to which the diagnostic assays it uses conform to the guidelines. (Strong consensus: 13/0/0)
8 Treatment of cutaneous Lyme borreliosis
Recommendations for treating Lyme borreliosis have appeared in numerous European and American guidelines since 2004 (see Annex 2 Comparison of Guidelines and Therapies in Attachment 1 [Att. 1]).
Table 5 [Tab. 5] summarises the best evaluated antibiotic therapies listed in American and European guidelines.
Table 5: Treatment recommendations for cutaneous Lyme borreliosis 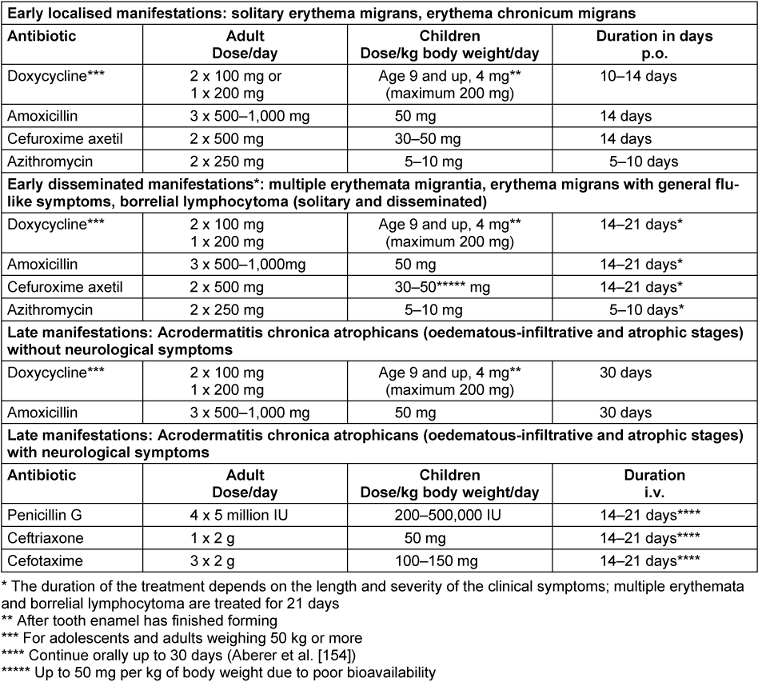
Doxycycline and Amoxicillin are the antibiotics of choice in all guidelines.
Both antibiotics are very effective in the dosages listed in Table 5 [Tab. 5] and are usually tolerated well [1], [142]. Gastrointestinal complaints can occur during doxycycline treatment. It is particularly important that doxycycline not be taken together with dairy products, dairy substitutes or drinks rich in Ca2+. Furthermore, patients should be informed of the risk of phototoxic skin reactions, particularly in the summer, and use sunscreen when taking the antibiotics.
During treatment with amoxicillin, non-allergenic skin exanthemas can frequently appear on the torso on day 8.
Treatment can continue if the exanthema is mild. If itching occurs, symptoms can be treated with antihistamines and skin care products. Corticosteroids are not necessary.
Of the oral cephalosporins, only cefuroxime axetil has demonstrated an efficacy that is comparable to that of doxycycline and amoxicillin [143]. The absolute bioavailability of cefuroxime axetil is comparatively low (40–45%). The best absorption is achieved when taken directly after a meal.
Other 1st and 2nd generation cephalosporins are not adequately effective [144].
In the case of an early disseminated infection, intravenous treatment with ceftriaxone does not achieve any better results than treatment with oral doxycycline [145].
Of the macrolides, azithromycin has proven to be sufficiently effective [1], [101], [142], [146], [147], [148]. The long tissue half-life period is advantageous because of the long generation time of Borrelia. The efficacy of clarithromycin is deemed controversial. Clarithromycin was compared to amoxicillin in a recent randomised open comparative study of children with erythema migrans and was found to be equally effective [1], [142], [149]. Roxithromycin is not adequately effective. Erythromycin is no longer the treatment of choice due to its unreliable absorption and indications of resistance [150].
Treatment with oral penicillin V is controversial. Austrian, Swedish and Slovenian studies show that it is adequately effective [101], [151], [152], [153]. Cochrane’s network meta-analysis has shown that oral penicillin V is just as effective as amoxicillin and doxycycline [1]. The dosage for adults is 3x1 million IU/day and for children 100,000 IU/kg/day for 14 days [101].
Minocycline is effective and authorised for the treatment of Lyme borreliosis. However, it cannot be recommended due to the increased risk of side-effects relating to the central nervous system and liver toxicity. Cochrane’s network meta-analysis has also shown that there is inadequate data, as there is only one comparative study of minocycline and penicillin V from 1996 [154].
It is particularly important that dose and treatment duration are adhered to.
Early cutaneous manifestations should be treated for 10–21 days (Table 5 [Tab. 5]). The length of treatment depends on the duration and severity of the clinical symptoms; 10–14 days of treatment is usually sufficient for solitary erythema migrans without general symptoms. In a comparative study, Stupica et al. [155] evaluated the outcome of treating localised erythema migrans with doxycycline for 10 versus 14 days. No difference was found in the treatment outcomes of the erythema. In both treatment groups, symptoms did not persist longer or more frequently after the end of treatment than in healthy subjects. Treatment should last 21 days if there are indications of Borrelia dissemination (such as flu-like symptoms), or in the case of multiple erythemata migrantia and borrelial lymphocytoma.
Oral doxycycline or amoxicillin treatment for 30 days is usually sufficient for late cutaneous manifestations (acrodermatitis chronica in the oedematous-infiltrative or atrophic stages) without neurological involvement [3], [91], [154]. If neurological symptoms are also present, intravenous treatment with penicillin G or the 3rd generation cephalosporins ceftriaxone or cefotaxime may be necessary (see the AWMF S3 guideline on neuroborreliosis [2]). To date there are no major controlled treatment studies on ACA.
The cure rate – defined as a restoration of the body to its original physical condition with regression of the disease-specific symptoms after successful treatment – is between 95% and 100% when the early localised and disseminated manifestations are treated in time [156], [157].
Treatment failures with pathogen detection after treatment are rare if treatment is carried out lege artis [54], [158]; isolated cases have been reported [121], [123], [159], [160], [161], [162]. Two major studies have shown that, in all cases, a new infection with another Borrelia strain was the cause of a renewed Lyme borreliosis infection [163], [164]. In a small case series, treatment failure was observed when there was immunosuppression with a TNF-α inhibitor [165].
In individual cases of late cutaneous manifestations where there is continued clinical and biopsy evidence of a persisting infection, treatment should be repeated with a different class of substance.
To date, there is no evidence of the development of secondary antibiotic resistance of B. burgdorferi to the antibiotics recommended in the guidelines [166], [167], [168], [169].
If the disease remains untreated for a longer period of time, there is an increased risk of persistent symptoms and defective healing of the skin, joints and nervous system increases [3].
It is a matter of controversy whether repeated antibiotic treatment makes sense for patients with persisting symptoms. According to published randomised controlled trials (RCT), long-term antibiotic treatment is less than promising [170], [171], [172], [173], [174], [175].
A European RCT that was published in 2016 (PLEASE Study) looked at 280 patients whose symptoms persisted for more than 2 years after their Lyme borreliosis was treated with antibiotics (78 patients after erythema migrans, 15 patients after meningoradiculitis) and 153 seropositive patients with borreliosis-related symptoms after a tick bite. The study compared the health-related effects of a 2-week versus 14-week round of antibiotics. Initially, all patients who had previously received antibiotic treatment received 2 g of ceftriaxone i.v. for 2 weeks. The patients were then randomly assigned to 3 groups. Group 1 received 200 mg/d of doxycycline p.o. for 12 weeks, group 2 received 2x500 mg of clarithromycin plus 2x200 mg/d of hydroxychloroquine for 12 weeks, and group 3 received a placebo for 12 weeks. Treatment success was based on a health-related quality of life after 14 weeks and then up to 52 weeks using the RAND 36 Health Status Inventory. The sum score improved equally after treatment in all 3 groups without significant differences; the assessment of the quality of life remained below that of the general population in all 3 groups. No difference in treatment success could be found between the short-term treatment and the two long-term treatments. Patients receiving the long-term treatment had considerably more antibiotic-related side-effects (primarily photosensitivity (18.6%) and nausea (10.5%) in connection with doxycycline, and primarily nausea (10.4%), diarrhoea (9.4%) and allergic exanthemas (8.3%) in connection with clarithromycin/hydroxychloroquine). Vision problems were most frequently reported in the placebo group (10% of patients) [176], [177].
8.1 Treatment during pregnancy and nursing
Oral treatment with amoxicillin p.o. is recommended during pregnancy and nursing. Alternatively, penicillin G and ceftriaxone can be administered i.v. [178], [179]. If the patient has a proven penicillin allergy, azithromycin or cefuroxime axetil can be prescribed after strong indication. Ceftriaxone can be administered intravenously under clinical supervision since, with 3rd generation cephalosporins, the risk of a cross allergy with penicillin is only around 1% [180].
8.2 Treating children
Children can be treated with doxycycline at a dose of 4 mg/kg BW/day up to a maximum dose of 200 mg/d from the age of 9 (>8 years) once enamel formation is complete. Even today, prescribing doxycycline to treat erythema migrans in children under 9 years of age is not recommended [181]. For children under 8, the treatment of choice is 50 mg/kg BW/d of amoxicillin (Table 5 [Tab. 5]). Taking it the required 3 times a day can be difficult for kindergarten- and school-aged children.
Alternatively, 30–50 mg/kg BW/d of cefuroxime axetil, 5–10 mg/kg BW/d of azithromycin, or 15 mg/kg BW/d of clarithromycin in two daily doses can be prescribed [149].
8.3 Treatment adherence
In order to improve treatment adherence/therapy compliance, the patient should be informed before beginning the treatment of the aspects of taking prescribed antibiotics and the potential risk of undesired effects.
A frequent cause of treatment failure is when doxycycline is taken incorrectly. It should be noted that absorption can be impaired by 2- or 3-valent cations such as aluminium, calcium (milk, dairy products and fruit juices containing calcium, water with a high mineral content), magnesium in antacids, iron preparations, as well as activated charcoal and cholestyramine. Therefore, there should be a 2-to-3-hour interval between taking the antibiotic and ingesting the medicine or food.
Another cause of treatment failure is when the antibiotic is taken irregularly e.g. skipping the midday dose in the case of amoxicillin, or not long enough e.g. due to a worsening of symptoms caused by a Herxheimer reaction, gastrointestinal complaints, or phototoxic skin reactions due to increased sensitivity to light from doxycycline. In addition, treatment failure was reported in a case series where there was immunosuppression with a TNF-α inhibitor [165].
In the case of a disseminated infection, patients should be informed about the possible occurrence of a Herxheimer reaction with a flare up of the erythema, which occurs in approx. 10% of cases. Approx. 2% of patients experience a feeling of unwellness and a rise in temperature within 24 hours of taking the antibiotics [88], [149]. Occasionally this reaction is delayed. This is a temporary immunological reaction caused by the upregulation of proinflammatory cytokines and can be treated, for example, with non-steroidal anti-inflammatory drugs (NSAID). Cortisone treatment is not necessary. The patient should continue to take the antibiotic.
Key statements and recommendations for the treatment of cutaneous Lyme borreliosis
Lyme borreliosis should be treated with antibiotics.
- Doxycycline or amoxicillin p.o. are the treatments of choice for cutaneous manifestations. (Consensus 13/0/1)
- Treatment alternatives for cutaneous manifestations are cefuroxime, azithromycin, possibly also clarithromycin p.o. (Strong consensus 14/0/0)
- See the recommendations of the other medical associations for the antibiotic treatment of patients with cutaneous Lyme borreliosis with neurological or cardiological manifestations. Ceftriaxone i.v., cefotaxime i.v., penicillin G i.v. or doxycycline p.o. may be considered. (Strong consensus 14/0/0)
- The treatment of early manifestations of cutaneous Lyme borreliosis should last 14–21 days (with the exception of azithromycin 5–10 days; doxycycline 10–14 days in the case of solitary erythema migrans). (Strong consensus 14/0/0)
- The treatment of late cutaneous manifestations should last 30 days. (Strong consensus 14/0/0)
- Antibiotic treatment should not generally extend beyond the recommended time period. (Consensus: 13/0/1)
- Treatment can be extended in individual cases depending on the clinical course of the disease and after re-evaluating the diagnosis. (Strong consensus: 14/0/0)
- Treatment should be repeated with a different class of substance in individual cases of late cutaneous manifestations if there is clinical or biopsy evidence of a persisting infection. (Consensus: 13/1/0)
- The diagnosis should be re-evaluated if the cutaneous symptoms persist or progress despite guideline-compliant antibiotic treatment. (Strong consensus: 14/0/0)
Recommendations for treating cutaneous Lyme borreliosis during pregnancy
- Amoxicillin p.o. is the treatment of choice during pregnancy. (Strong consensus: 14/0/0)
- Treatment alternatives during pregnancy or while nursing are penicillin G i.v. or ceftriaxone i.v. (Strong consensus: 14/0/0)
- Cefuroxime p.o., ceftriaxone i.v., cefotaxime i.v. or azithromycin p.o. should be prescribed if the patient is allergic to penicillin. (Strong consensus: 14/0/0)
Treatment recommendation for cutaneous Lyme borreliosis in children
- Amoxicillin p.o. is the treatment of choice for children under 8 years of age. (Strong consensus: 14/0/0)
- Children 9 years and older can receive doxycycline p.o. (Strong consensus: 14/0/0)
- Azithromycin, clarithromycin or cefuroxime p.o. are treatment alternatives for children. (Strong consensus: 14/0/0)
See the guideline report for the dissenting opinion of the Borreliosis and FSME Association of Germany on treatment [25].
8.4 Persisting symptoms after treatment/post-treatment Lyme disease syndrome (PTLDS)
Inflammatory reactions can persist and symptoms such as fatigue, joint and muscle pain, headaches, a general feeling of unwellness, irritability or paraesthesia can last for months, even following guideline-compliant antibiotic treatment. If the non-specific symptoms persist for more than 6 months, this is referred to by some authors as Post Lyme Syndrome (PLS) or Posttreatment Lyme Disease Syndrome (PTLDS) [173], [182]. So-called PTLDS is a syndrome that has yet to be generally defined scientifically and is, therefore, not yet universally accepted. It can be differentiated diagnostically from confirmed late manifestations of Lyme disease, symptoms resulting from the persistence of reproducible pathogens, and dysfunctional healing. Repeated and long-term antibiotic treatment does not provide any proven benefit [183], but is associated with the risk of considerable side effects, some of which can be life-threatening [170], [171], [172], [174]. The issue of PTLDS is discussed in detail in the S3 guideline on neuroborreliosis [2] (online: https://www.dgn.org/leitlinien).
A controlled study of patients with erythema migrans, in which an age- and sex-matched control group was simultaneously studied, found that there was no increased incidence of post-therapeutic symptoms compared to the control group [156].
It is crucial that the complaints of patients with new or persisting symptoms after guideline-compliant antibiotic treatment are taken seriously, further diagnostic testing is performed on a case-by-case basis, and that any further treatment is carried out after careful consideration of the risks and benefits [183]. The need for research into PTLDS remains exceedingly high.
8.5 Procedure when skin changes and symptoms persist after antibiotic treatment
A false primary diagnosis is a common reason why there are persisting skin changes and symptoms following antibiotic treatment 2009 [184].
In the case of a clinically diagnosed erythema migrans and multiple erythema migrantia that do not heal within 6 weeks, a differential diagnosis of circumscribed scleroderma (morphea), granuloma annulare, sarcoidosis, erythema annulare et diutinum, tinea (with low epidermal involvement) or urticarial vasculitis should be considered. Patients should be referred to a dermatologist for further diagnostic testing. Borrelial lymphocytoma often heals very slowly over many months. According to investigations by Maraspin et al. on 85 patients, the average healing time was 28 days (7–270 days). The longer the borrelial lymphocytoma was present, the longer it took to heal [81]. If nodules persist for more than 1 year or if new nodules appear, a skin biopsy should be performed by a dermatologist for histological analysis and a Borrelia PCR. The differential diagnosis includes a cutaneous pseudolymphoma, a Jessner-Kanof lymphocytic infiltration, or a malignant lymphoma.
It takes years following antibiotic treatment for skin changes to slowly regress in cases of acrodermatitis chronica atrophicans that have persisted for years. Atrophies of the skin, tissue and fat can be irreversible – especially in older people. This also applies to ACA-associated peripheral neuropathy. (See also the AWMF-S3 Guideline on Neuroborreliosis [2].)
The differential diagnosis includes age-related skin atrophy, chronic thermal damage to the skin, e.g. chilblains and heat melanosis, as well as chronic venous insufficiency with stasis dermatitis.
Chronic neuropathic pain after adequate antibiotic treatment of acrodermatitis chronica atrophicans with peripheral neuropathy is treated in accordance with the DGN’s guideline on neuropathic pain (AWMF – guideline register no. 030/114 [185]).
All patients with persisting symptoms that cannot be attributed to cutaneous Lyme borreliosis or can no longer be attributed to cutaneous Lyme borreliosis after antibiotic treatment, should undergo careful differential diagnosis by an appropriate specialist, above all for internal medicine (infectiology, rheumatology, cardiology, endocrinology) as well as for neurology, psychosomatics and psychotherapy, psychiatry and pain therapy, since chronic infectious diseases of a different aetiology, other internal diseases, autoimmune diseases, chronic pain syndromes, depressive and somatoform or somatic stress disorders may also be considered in the differential diagnosis and must be treated accordingly (AWMF S3 Leitlinie “Funktionelle Körperbeschwerden” [186]; [2], [183], [187]).
There are no clinical studies that specifically look at the (symptomatic) treatment of persisting symptoms following adequate antibiotic treatment of Lyme borreliosis.
Recommendations for persisting symptoms following guideline-compliant treatment
If an erythema treated as erythema migrans or multiple erythema persists (longer than 6 weeks), the patient shall be referred to a dermatologist for a differential diagnosis of circumscribed scleroderma (morphea), granuloma annulare, sarcoidosis, erythema annulare et diutinum, tinea or urticarial vasculitis. (Strong consensus: 12/0/0)
If a lymphocytoma persists or progresses after treatment, the patient shall be referred to a dermatologist for a differential diagnosis (cutaneous pseudolymphoma, Jessner’s lymphocytic infiltration or malignant lymphoma). (Strong consensus: 12/0/0)
If an acrodermatitis chronica atrophicans persists after treatment, the patient shall be referred to a dermatologist for further consultation and for a differential diagnosis (age-related skin atrophy, chronic thermal damage to the skin e.g. chilblains and heat melanosis, chronic venous insufficiency with stasis dermatitis). (Strong consensus: 12/0/0)
9 Prophylaxis
9.1 Preventing tick bites
The best prophylaxis is to prevent tick bites by wearing clothing that covers the body and to carefully check the skin, including the scalp under the hair, after spending time outdoors. This is particularly important for children, who are at increased risk when playing outdoors between spring and autumn.
Insect repellents that are effective against ticks, e.g. diethyl-meta-toluamide (DEET), icaridin (1-(1-methylpropyl carbonyl)-2-(2-hydroxyethyl)piperidine) and ethyl butylacetylaminopropionate (EBAAP, IR 3535) can also be used, but only have a limited effectiveness of up to 4 hours [188], [189], [190].
Clothing that is impregnated with permethrin is also available, although no studies are available on its ability to repel I. ricinus or its long-term effects on humans.
9.2 Preventing Lyme borreliosis
It is very important to remove ticks early, before they have become engorged with blood. The risk of Borrelia transmission increases with the length of the time the tick feeds [24]. The time the I. ricinus needs to feed before there is a risk of transmission to humans has not yet been sufficiently investigated. It is important to emphasise that results from the USA, which assume transmission after at least 24 hours of feeding – but with a different vector and only B. burgdorferi s.s. – are not transferable to Europe. Therefore, every tick bite must be regarded in principle as potentially infectious, even if the probability of transmission increases with the length of time of the feeding act.
After spending time in a garden, park, field, forest or meadow where contact with ticks is possible, the body should be checked the same evening for ticks. Showering alone is not enough. The tick often remains on the body while it searches for a suitable location, e.g. back of the knees, armpits, between the toes, groin, genitals, in children often under the hair on the scalp, or neck.
Ticks should be removed immediately using tick tweezers or tick cards to prevent the transmission of Borrelia. If parts of the feeding apparatus remain in the skin, they can be removed later using a needle or curettage [182]. If the head or the feeding apparatus remains in the skin, there is no harm with regard to the transmission of the Borrelia. In the case of fully engorged nymphs and adult ticks, the body of the tick should not be squeezed in order to prevent the possible transmission of the Borrelia.
Examining a tick that has been removed from the skin for Borrelia is not useful, as the detection of Borrelia in the tick is not sufficiently predictive of the transmission of the Borrelia to the host and the development of disease.
After the removal of a tick, patients should be informed about the need for observing, in particular, the bite site over the next 6 weeks (Annex 1: Patient information after a tick bite in Attachment 1 [Att. 1]).
9.3 Prophylactic treatment after a tick bite
According to an American study, the risk of infection can be reduced by taking a one-time prophylactic dose of 200 mg doxycycline after a tick bite (effectiveness of 87%) [191], [192]. However, the findings should be interpreted with caution, as only one follow-up took place after 6 weeks. Thus, no statement can be made about whether this is sufficiently effective with respect to late infections.
A recent European study – an open-label RCT with participation via the internet – looked at 1,689 subjects. It analysed participants >8 years of age, doxycycline 1x200 mg within 72 h after tick removal, no tick bite before or in the following 3 months, completed questionnaire after 1 week, 1 month and then every 3 months up to 18 months and no evidence of Lyme borreliosis when entering the study. A total of 29 cases (28 erythema migrans and one acrodermatitis chronica atrophicans) were found, 10 out of 1,041 (0.96%) in the prophylaxis group and 19 out of 648 (2.9%) in the group without prophylaxis. This results in a number needed to treat (NNT) of 51 (95%-CI 29–180) to prevent one case of Lyme borreliosis, corresponding to a relative risk reduction of 67% (95%-CI 31–84%) [193]. The NNT could be reduced to around 10 when other parameters were included, such as the tick’s feeding conditions or a Borrelia PCR from the tick, but such procedures – including tick removal, dispatch to a laboratory with the appropriate expertise, phenotypic and molecular tests – in order to receive a prophylaxis within 72 h is not feasible. The authors conclude that prophylaxis in Europe is as effective as in the USA, that internet studies allow larger numbers of participants and that the inclusion of tick properties in the decision-making process could further increase efficacy.
In light of the low risk of infection, doxycycline would have to be unnecessarily administered many times in order to prevent a potential infection. According to projections for infection risk in endemic areas, 40–125 prophylaxes would have to be administered to prevent 1 infection [194]. Impact on the intestinal flora and possible development of resistance through the frequent administration of prophylaxes is conceivable. For this reason, oral doxycycline prophylaxis is not recommended in Europe.
The prophylactic use of antibiotic creams is also controversial. Animal studies with azithromycin cream reveal good prophylactic efficacy [195], [196]. A placebo-controlled study on efficacy in humans did not find sufficient efficacy, which is why this prophylactic measure cannot be recommended at present [197].
Recommendations for infection prophylaxis
Clothing that covers the body should be worn to prevent tick bites.
- The use of tick repellents can be recommended to a limited degree.
- After spending time outdoors where contact with ticks is possible, the skin should be checked for ticks no later than that evening.
- Ticks should be removed early to prevent Lyme borreliosis.
- The site of the bite should be observed for up to six weeks.
(Consensus: 12/0/1)
Not recommended
- The removed tick should not be analysed for Borrelia (Consensus: 12/0/1)
- Local or systemic prophylactic antibiotic treatment after a tick bite should not be carried out. (Consensus: 12/0/1)
9.4 Vaccines
Currently there is no vaccine approved for use in humans.
A vaccine with lipidated recombinant Osp A was evaluated as part of large-scale studies in the USA and was found to have good efficacy [198], [199]. The vaccine was approved in the USA in 1999 but was withdrawn from the market by the manufacturer in 2002. The reasons for this are not of a medical nature. Reports of adverse vaccine reactions in genetically predisposed individuals have been refuted by several qualified studies [200], [201], [202]. This monovalent vaccine is not suitable for Europe as it only protects against infection with B. burgdorferi sensu stricto and not against the genospecies B. afzelii and B. garinii, which are frequently found in Europe. A polyvalent OspA vaccine is currently being developed for Europe [203], however approval is not expected in the foreseeable future.
Notes
Guideline information
This is the English version of the German DGN S2k Guideline “Kutane Lyme Borreliose”, AWMF Register Number: 013/044 [204].
Disclaimer
The “guidelines” of the scientific medical societies are a systematically developed aid for physicians to reach decisions in specific situations. They are based on current scientific knowledge and good practice and ensure greater safety in medicine. They also take economic factors into consideration. The “guidelines” are not legally binding for physicians and therefore have neither a liability or liability releasing function.
The AWMF prepares and publishes the guidelines of the medical societies with great care – however the AWMF assumes no liability for accuracy – particularly with regard to dosage information.
Procedure for forming a consensus
The guideline was drawn up using a modified Delphi process and voted on in an extended consensus conference of the Interdisciplinary S3 Guideline Group, moderated by Prof Ina Kopp, Head of the AWMF Institute for Medical Knowledge Management.
It was adopted by the 22 participating medical societies and patient organisations.
Competing interests
See Attachment 2 [Att. 2].
References
[1] Torbahn G, Hofmann H, Rücker G, Bischoff K, Freitag MH, Dersch R, Fingerle V, Motschall E, Meerpohl JJ, Schmucker C. Efficacy and Safety of Antibiotic Therapy in Early Cutaneous Lyme Borreliosis: A Network Meta-analysis. JAMA Dermatol. 2018 Nov 1;154(11):1292-303. DOI: 10.1001/jamadermatol.2018.3186[2] Deutsche Gesellschaft für Neurologie e.V. (DGN). S3-Leitlinie Neuroborreliose. Version 6.0. AWMF-Register-Nr. 030-071. Berlin: AWMF; 2024. Available from: https://register.awmf.org/de/leitlinien/detail/030-071
[3] Ogrinc K, Maraspin V, Lusa L, Cerar Kišek T, Ružić-Sabljić E, Strle F. Acrodermatitis chronica atrophicans: clinical and microbiological characteristics of a cohort of 693 Slovenian patients. J Intern Med. 2021 Aug;290(2):335-48. DOI: 10.1111/joim.13266
[4] Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti JC, Assous M, Grimont PA. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992 Jul;42(3):378-83. DOI: 10.1099/00207713-42-3-378
[5] Schaarschmidt D, Oehme R, Kimmig P, Hesch RD, Englisch S. Detection and molecular typing of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks and in different patient samples from southwest Germany. Eur J Epidemiol. 2001;17(12):1067-74. DOI: 10.1023/a:1021286528058
[6] Richter D, Postic D, Sertour N, Livey I, Matuschka FR, Baranton G. Delineation of Borrelia burgdorferi sensu lato species by multilocus sequence analysis and confirmation of the delineation of Borrelia spielmanii sp. nov. Int J Syst Evol Microbiol. 2006 Apr;56(Pt 4):873-81. DOI: 10.1099/ijs.0.64050-0
[7] Fingerle V, Schulte-Spechtel UC, Ruzic-Sabljic E, Leonhard S, Hofmann H, Weber K, Pfister K, Strle F, Wilske B. Epidemiological aspects and molecular characterization of Borrelia burgdorferi s.l. from southern Germany with special respect to the new species Borrelia spielmanii sp. nov. Int J Med Microbiol. 2008 Apr;298(3-4):279-90. DOI: 10.1016/j.ijmm.2007.05.002
[8] Wallich R, Helmes C, Schaible UE, Lobet Y, Moter SE, Kramer MD, Simon MM. Evaluation of genetic divergence among Borrelia burgdorferi isolates by use of OspA, fla, HSP60, and HSP70 gene probes. Infect Immun. 1992 Nov;60(11):4856-66. DOI: 10.1128/iai.60.11.4856-4866.1992
[9] Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, Krause PJ. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011 Oct;17(10):1816-23. DOI: 10.3201/eid1710.101474
[10] Stanek G, Strle F. Lyme borreliosis. Lancet. 2003 Nov 15;362(9396):1639-47. DOI: 10.1016/S0140-6736(03)14798-8
[11] Maraspin V, Ruzic-Sabljic E, Strle F. Lyme borreliosis and Borrelia spielmanii. Emerg Infect Dis. 2006 Jul;12(7):1177. DOI: 10.3201/eid1207.060077
[12] Berglund J, Eitrem R, Ornstein K, Lindberg A, Ringér A, Elmrud H, Carlsson M, Runehagen A, Svanborg C, Norrby R. An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med. 1995 Nov 16;333(20):1319-27. DOI: 10.1056/NEJM199511163332004
[13] Huppertz HI, Böhme M, Standaert SM, Karch H, Plotkin SA. Incidence of Lyme borreliosis in the Würzburg region of Germany. Eur J Clin Microbiol Infect Dis. 1999 Oct;18(10):697-703. DOI: 10.1007/s100960050381
[14] Enkelmann J, Böhmer M, Fingerle V, Siffczyk C, Werber D, Littmann M, Merbecks SS, Helmeke C, Schroeder S, Hell S, Schlotthauer U, Burckhardt F, Stark K, Schielke A, Wilking H. Incidence of notified Lyme borreliosis in Germany, 2013-2017. Sci Rep. 2018 Oct 8;8(1):14976. DOI: 10.1038/s41598-018-33136-0
[15] Wilking H, Stark K. Trends in surveillance data of human Lyme borreliosis from six federal states in eastern Germany, 2009-2012. Ticks Tick Borne Dis. 2014 Apr;5(3):219-24. DOI: 10.1016/j.ttbdis.2013.10.010
[16] Müller I, Freitag MH, Poggensee G, Scharnetzky E, Straube E, Schoerner Ch, Hlobil H, Hagedorn HJ, Stanek G, Schubert-Unkmeir A, Norris DE, Gensichen J, Hunfeld KP. Evaluating frequency, diagnostic quality, and cost of Lyme borreliosis testing in Germany: a retrospective model analysis. Clin Dev Immunol. 2012;2012:595427. DOI: 10.1155/2012/595427
[17] Akmatov MK, Holstiege J, Dammertz L, Kohring C, Heuer J, Bätzing J. Bundesweite und kleinräumige Kennzahlen zur Morbidität von Lyme-Borreliose in Deutschland anhand Vertragsärztlicher Abrechnungsdaten, 2010 bis 2019. Versorgungsatlas-Bericht. 2021;Bericht Nr. 21/06:1-18. DOI: 10.20364/VA-21.06
[18] Wilking H, Fingerle V, Klier C, Thamm M, Stark K. Antibodies against Borrelia burgdorferi sensu lato among Adults, Germany, 2008-2011. Emerg Infect Dis. 2015 Jan;21(1):107-10. DOI: 10.3201/eid2101.140009
[19] Woudenberg T, Böhm S, Böhmer M, Katz K, Willrich N, Stark K, Kuhnert R, Fingerle V, Wilking H. Dynamics of Borrelia burgdorferi-Specific Antibodies: Seroconversion and Seroreversion between Two Population-Based, Cross-Sectional Surveys among Adults in Germany. Microorganisms. 2020 Nov 25;8(12):1859. DOI: 10.3390/microorganisms8121859
[20] Wilhelmsson P, Fryland L, Lindblom P, Sjöwall J, Ahlm C, Berglund J, Haglund M, Henningsson AJ, Nolskog P, Nordberg M, Nyberg C, Ornstein K, Nyman D, Ekerfelt C, Forsberg P, Lindgren PE. A prospective study on the incidence of Borrelia burgdorferi sensu lato infection after a tick bite in Sweden and on the Åland Islands, Finland (2008-2009). Ticks Tick Borne Dis. 2016 Feb;7(1):71-9. DOI: 10.1016/j.ttbdis.2015.08.009
[21] Nigrovic LE, Neville DN, Balamuth F, Bennett JE, Levas MN, Garro AC; for Pedi Lyme Net. A minority of children diagnosed with Lyme disease recall a preceding tick bite. Ticks Tick Borne Dis. 2019 Apr;10(3):694-96. DOI: 10.1016/j.ttbdis.2019.02.015
[22] Fingerle V, Bergmeister H, Liegl G, Vanek E, Wilske B. Prevalence of Borrelia burgdorferi sensu lato in Ixodes ricinus in Southern Germany. J Spiroch Tick Dis. 1994;(1):41-5.
[23] Cotté V, Sabatier L, Schnell G, Carmi-Leroy A, Rousselle JC, Arsène-Ploetze F, Malandrin L, Sertour N, Namane A, Ferquel E, Choumet V. Differential expression of Ixodes ricinus salivary gland proteins in the presence of the Borrelia burgdorferi sensu lato complex. J Proteomics. 2014 Jan 16;96:29-43. DOI: 10.1016/j.jprot.2013.10.033
[24] Gern L. Life cycle of Borrelia burgdorferi sensu lato and transmission to humans. Curr Probl Dermatol. 2009;37:18-30. DOI: 10.1159/000213068
[25] Deutsche Dermatologische Gesellschaft e.V. (DDG). Leitlinienreport der S2k-Leitlinie Kutane Lyme Borreliose. Version 3.0. AWMF-Registernummer 013-044. Berlin: AWMF; 2023. Available from: https://register.awmf.org/de/leitlinien/detail/013-044
[26] Hartiala P, Hytönen J, Suhonen J, Leppäranta O, Tuominen-Gustafsson H, Viljanen MK. Borrelia burgdorferi inhibits human neutrophil functions. Microbes Infect. 2008 Jan;10(1):60-8. DOI: 10.1016/j.micinf.2007.10.004
[27] de Taeye SW, Kreuk L, van Dam AP, Hovius JW, Schuijt TJ. Complement evasion by Borrelia burgdorferi: it de Taeye SW, Kreuk L, van Dam AP, Hovius JW, Schuijt TJ. Complement evasion by Borrelia burgdorferi: it takes three to tango. Trends Parasitol. 2013 Mar;29(3):119-28. DOI: 10.1016/j.pt.2012.12.001
[28] Rogovskyy AS, Bankhead T. Variable VlsE is critical for host reinfection by the Lyme disease spirochete. PLoS One. 2013 Apr 8;8(4):e61226. DOI: 10.1371/journal.pone.0061226
[29] Abi ME, Ji Z, Jian M, Dai X, Bai R, Ding Z, Luo L, Chen T, Wang F, Wen S, Zhou G, Bao F, Liu A. Molecular Interactions During Borrelia burgdorferi Migration from the Vector to the Mammalian Nervous System. Curr Protein Pept Sci. 2020;21(5):517-26. DOI: 10.2174/1389203720666191015145714
[30] Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002 Jul 1;169(1):10-4. DOI: 10.4049/jimmunol.169.1.10
[31] Cruz AR, Moore MW, La Vake CJ, Eggers CH, Salazar JC, Radolf JD. Phagocytosis of Borrelia burgdorferi, the Lyme disease spirochete, potentiates innate immune activation and induces apoptosis in human monocytes. Infect Immun. 2008 Jan;76(1):56-70. DOI: 10.1128/IAI.01039-07
[32] Hovius JW, Bijlsma MF, van der Windt GJ, Wiersinga WJ, Boukens BJ, Coumou J, Oei A, de Beer R, de Vos AF, van 't Veer C, van Dam AP, Wang P, Fikrig E, Levi MM, Roelofs JJ, van der Poll T. The urokinase receptor (uPAR) facilitates clearance of Borrelia burgdorferi. PLoS Pathog. 2009 May;5(5):e1000447. DOI: 10.1371/journal.ppat.1000447
[33] Petzke MM, Brooks A, Krupna MA, Mordue D, Schwartz I. Recognition of Borrelia burgdorferi, the Lyme disease spirochete, by TLR7 and TLR9 induces a type I IFN response by human immune cells. J Immunol. 2009 Oct 15;183(8):5279-92. DOI: 10.4049/jimmunol.0901390
[34] Salazar JC, Duhnam-Ems S, La Vake C, Cruz AR, Moore MW, Caimano MJ, Velez-Climent L, Shupe J, Krueger W, Radolf JD. Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-beta. PLoS Pathog. 2009 May;5(5):e1000444. DOI: 10.1371/journal.ppat.1000444
[35] Bachmann M, Horn K, Rudloff I, Goren I, Holdener M, Christen U, Darsow N, Hunfeld KP, Koehl U, Kind P, Pfeilschifter J, Kraiczy P, Mühl H. Early production of IL-22 but not IL-17 by peripheral blood mononuclear cells exposed to live Borrelia burgdorferi: the role of monocytes and interleukin-1. PLoS Pathog. 2010 Oct 14;6(10):e1001144. DOI: 10.1371/journal.ppat.1001144
[36] Skogman BH, Hellberg S, Ekerfelt C, Jenmalm MC, Forsberg P, Ludvigsson J, Bergström S, Ernerudh J. Adaptive and innate immune responsiveness to Borrelia burgdorferi sensu lato in exposed asymptomatic children and children with previous clinical Lyme borreliosis. Clin Dev Immunol. 2012;2012:294587. DOI: 10.1155/2012/294587
[37] Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012 Feb 4;379(9814):461-73. DOI: 10.1016/S0140-6736(11)60103-7
[38] Ribeiro JM, Makoul GT, Levine J, Robinson DR, Spielman A. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J Exp Med. 1985 Feb 1;161(2):332-44. DOI: 10.1084/jem.161.2.332
[39] Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, Anguita J, Norgard MV, Kantor FS, Anderson JF, Koski RA, Fikrig E. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005 Jul 28;436(7050):573-7. DOI: 10.1038/nature03812
[40] Hovius JW, Schuijt TJ, de Groot KA, Roelofs JJ, Oei GA, Marquart JA, de Beer R, van 't Veer C, van der Poll T, Ramamoorthi N, Fikrig E, van Dam AP. Preferential protection of Borrelia burgdorferi sensu stricto by a Salp15 homologue in Ixodes ricinus saliva. J Infect Dis. 2008 Oct 15;198(8):1189-97. DOI: 10.1086/591917
[41] Dai J, Wang P, Adusumilli S, Booth CJ, Narasimhan S, Anguita J, Fikrig E. Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell Host Microbe. 2009 Nov 19;6(5):482-92. DOI: 10.1016/j.chom.2009.10.006
[42] Horká H, Cerná-Kýcková K, Skallová A, Kopecký J. Tick saliva affects both proliferation and distribution of Borrelia burgdorferi spirochetes in mouse organs and increases transmission of spirochetes to ticks. Int J Med Microbiol. 2009 Jun;299(5):373-80. DOI: 10.1016/j.ijmm.2008.10.009
[43] Dai J, Narasimhan S, Zhang L, Liu L, Wang P, Fikrig E. Tick histamine release factor is critical for Ixodes scapularis engorgement and transmission of the lyme disease agent. PLoS Pathog. 2010 Nov 24;6(11):e1001205. DOI: 10.1371/journal.ppat.1001205
[44] Kern A, Collin E, Barthel C, Michel C, Jaulhac B, Boulanger N. Tick saliva represses innate immunity and cutaneous inflammation in a murine model of Lyme disease. Vector Borne Zoonotic Dis. 2011 Oct;11(10):1343-50. DOI: 10.1089/vbz.2010.0197
[45] Oliveira CJ, Sá-Nunes A, Francischetti IM, Carregaro V, Anatriello E, Silva JS, Santos IK, Ribeiro JM, Ferreira BR. Deconstructing tick saliva: non-protein molecules with potent immunomodulatory properties. J Biol Chem. 2011 Apr 1;286(13):10960-9. DOI: 10.1074/jbc.M110.205047
[46] Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012 Jan 9;10(2):87-99. DOI: 10.1038/nrmicro2714
[47] Mullegger RR. Dermatological manifestations of Lyme borreliosis. Eur J Dermatol. 2004 Sep-Oct;14(5):296-309.
[48] Strle K, Stupica D, Drouin EE, Steere AC, Strle F. Elevated levels of IL-23 in a subset of patients with post-lyme disease symptoms following erythema migrans. Clin Infect Dis. 2014 Feb;58(3):372-80. DOI: 10.1093/cid/cit735
[49] Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, Rosa P. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun. 2006 Jun;74(6):3554-64. DOI: 10.1128/IAI.01950-05
[50] Bankhead T, Chaconas G. The role of VlsE antigenic variation in the Lyme disease spirochete: persistence through a mechanism that differs from other pathogens. Mol Microbiol. 2007 Sep;65(6):1547-58. DOI: 10.1111/j.1365-2958.2007.05895.x
[51] Xu Q, McShan K, Liang FT. Identification of an ospC operator critical for immune evasion of Borrelia burgdorferi. Mol Microbiol. 2007 Apr;64(1):220-31. DOI: 10.1111/j.1365-2958.2007.05636.x
[52] Crowley JT, Toledo AM, LaRocca TJ, Coleman JL, London E, Benach JL. Lipid exchange between Borrelia burgdorferi and host cells. PLoS Pathog. 2013 Jan;9(1):e1003109. DOI: 10.1371/journal.ppat.1003109
[53] Hartmann K, Corvey C, Skerka C, Kirschfink M, Karas M, Brade V, Miller JC, Stevenson B, Wallich R, Zipfel PF, Kraiczy P. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol Microbiol. 2006 Sep;61(5):1220-36. DOI: 10.1111/j.1365-2958.2006.05318.x
[54] Hunfeld KP, Ruzić-Sabljić E, Norris DE, Kraiczy P, Strle F. Risk of culture-confirmed borrelial persistence in patients treated for erythema migrans and possible mechanisms of resistance. Int J Med Microbiol. 2006 May;296 Suppl 40:233-41. DOI: 10.1016/j.ijmm.2006.01.028
[55] Kraiczy P, Rossmann E, Brade V, Simon MM, Skerka C, Zipfel PF, Wallich R. Binding of human complement regulators FHL-1 and factor H to CRASP-1 orthologs of Borrelia burgdorferi. Wien Klin Wochenschr. 2006 Nov;118(21-22):669-76. DOI: 10.1007/s00508-006-0691-1
[56] Kraiczy P, Würzner R. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol Immunol. 2006 Jan;43(1-2):31-44. DOI: 10.1016/j.molimm.2005.06.016
[57] Rossmann E, Kitiratschky V, Hofmann H, Kraiczy P, Simon MM, Wallich R. Borrelia burgdorferi complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes is expressed in humans and induces antibody responses restricted to nondenatured structural determinants. Infect Immun. 2006 Dec;74(12):7024-8. DOI: 10.1128/IAI.01028-06
[58] Fuchs H, Simon MM, Wallich R, Bechtel M, Kramer MD. Borrelia burgdorferi induces secretion of pro-urokinase-type plasminogen activator by human monocytes. Infect Immun. 1996 Oct;64(10):4307-12. DOI: 10.1128/iai.64.10.4307-4312.1996
[59] Grab DJ, Kennedy R, Philipp MT. Borrelia burgdorferi possesses a collagenolytic activity. FEMS Microbiol Lett. 1996 Oct 15;144(1):39-45. DOI: 10.1111/j.1574-6968.1996.tb08506.x
[60] Coleman JL, Roemer EJ, Benach JL. Plasmin-coated borrelia Burgdorferi degrades soluble and insoluble components of the mammalian extracellular matrix. Infect Immun. 1999 Aug;67(8):3929-36. DOI: 10.1128/IAI.67.8.3929-3936.1999
[61] Önder Ö, Humphrey PT, McOmber B, Korobova F, Francella N, Greenbaum DC, Brisson D. OspC is potent plasminogen receptor on surface of Borrelia burgdorferi. J Biol Chem. 2012 May 11;287(20):16860-8. DOI: 10.1074/jbc.M111.290775
[62] Shi Y, Xu Q, Seemanaplli SV, McShan K, Liang FT. Common and unique contributions of decorin-binding proteins A and B to the overall virulence of Borrelia burgdorferi. PLoS One. 2008 Oct 3;3(10):e3340. DOI: 10.1371/journal.pone.0003340
[63] Srivastava SY, de Silva AM. Reciprocal expression of ospA and ospC in single cells of Borrelia burgdorferi. J Bacteriol. 2008 May;190(10):3429-33. DOI: 10.1128/JB.00085-08
[64] Xu Q, McShan K, Liang FT. Essential protective role attributed to the surface lipoproteins of Borrelia burgdorferi against innate defences. Mol Microbiol. 2008 Jul;69(1):15-29. DOI: 10.1111/j.1365-2958.2008.06264.x
[65] Sarkar A, Tilly K, Stewart P, Bestor A, Battisti JM, Rosa PA. Borrelia burgdorferi resistance to a major skin antimicrobial peptide is independent of outer surface lipoprotein content. Antimicrob Agents Chemother. 2009 Oct;53(10):4490-4. DOI: 10.1128/AAC.00558-09
[66] Tilly K, Bestor A, Dulebohn DP, Rosa PA. OspC-independent infection and dissemination by host-adapted Borrelia burgdorferi. Infect Immun. 2009 Jul;77(7):2672-82. DOI: 10.1128/IAI.01193-08
[67] Rupprecht TA, Kirschning CJ, Popp B, Kastenbauer S, Fingerle V, Pfister HW, Koedel U. Borrelia garinii induces CXCL13 production in human monocytes through Toll-like receptor 2. Infect Immun. 2007 Sep;75(9):4351-6. DOI: 10.1128/IAI.01642-06
[68] Shin OS, Isberg RR, Akira S, Uematsu S, Behera AK, Hu LT. Distinct roles for MyD88 and Toll-like receptors 2, 5, and 9 in phagocytosis of Borrelia burgdorferi and cytokine induction. Infect Immun. 2008 Jun;76(6):2341-51. DOI: 10.1128/IAI.01600-07
[69] Yang X, Izadi H, Coleman AS, Wang P, Ma Y, Fikrig E, Anguita J, Pal U. Borrelia burgdorferi lipoprotein BmpA activates pro-inflammatory responses in human synovial cells through a protein moiety. Microbes Infect. 2008 Oct;10(12-13):1300-8. DOI: 10.1016/j.micinf.2008.07.029
[70] Gautam A, Dixit S, Philipp MT, Singh SR, Morici LA, Kaushal D, Dennis VA. Interleukin-10 alters effector functions of multiple genes induced by Borrelia burgdorferi in macrophages to regulate Lyme disease inflammation. Infect Immun. 2011 Dec;79(12):4876-92. DOI: 10.1128/IAI.05451-11
[71] Lee WY, Moriarty TJ, Wong CH, Zhou H, Strieter RM, van Rooijen N, Chaconas G, Kubes P. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol. 2010 Apr;11(4):295-302. DOI: 10.1038/ni.1855
[72] Shen S, Shin JJ, Strle K, McHugh G, Li X, Glickstein LJ, Drouin EE, Steere AC. Treg cell numbers and function in patients with antibiotic-refractory or antibiotic-responsive Lyme arthritis. Arthritis Rheum. 2010 Jul;62(7):2127-37. DOI: 10.1002/art.27468
[73] Codolo G, Bossi F, Durigutto P, Bella CD, Fischetti F, Amedei A, Tedesco F, D'Elios S, Cimmino M, Micheletti A, Cassatella MA, D'Elios MM, de Bernard M. Orchestration of inflammation and adaptive immunity in Borrelia burgdorferi-induced arthritis by neutrophil-activating protein A. Arthritis Rheum. 2013 May;65(5):1232-42. DOI: 10.1002/art.37875
[74] Strle F, Lusa L, Ružić-Sabljić E, Maraspin V, Lotrič Furlan S, Cimperman J, Ogrinc K, Rojko T, Videčnik Zorman J, Stupica D. Clinical characteristics associated with Borrelia burgdorferi sensu lato skin culture results in patients with erythema migrans. PLoS One. 2013 Dec 26;8(12):e82132. DOI: 10.1371/journal.pone.0082132
[75] Stanek G, Fingerle V, Hunfeld KP, Jaulhac B, Kaiser R, Krause A, Kristoferitsch W, O'Connell S, Ornstein K, Strle F, Gray J. Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect. 2011 Jan;17(1):69-79. DOI: 10.1111/j.1469-0691.2010.03175.x
[76] Hofmann H. Variabilität der kutanen Lyme-Borreliose. Diagnostik und Therapie [The variable spectrum of cutaneous Lyme borreliosis. Diagnosis and therapy]. Hautarzt. 2012 May;63(5):381-9. DOI: 10.1007/s00105-011-2256-0
[77] Eriksson P, Schröder MT, Niiranen K, Nevanlinna A, Panelius J, Ranki A. The many faces of solitary and multiple erythema migrans. Acta Derm Venereol. 2013 Nov;93(6):693-700. DOI: 10.2340/00015555-1549
[78] Aberer E. Lyme borreliosis--an update. J Dtsch Dermatol Ges. 2007 May;5(5):406-14. DOI: 10.1111/j.1610-0387.2007.06285.x
[79] Oschmann P, Kraiczy P, Halperin J, Brade V. Lyme borreliosis and tick-borne encephalitis. UNI-MED Verlag; 1999.
[80] Ang CW, Brandenburg AH, van Burgel ND, Bijlmer HA, Herremans T, Stelma F, Lunel FV, van Dam AP; Dutch Working Group on Diagnosis of Lyme Borreliosis. A Dutch nationwide evaluation of serological assays for detection of Borrelia antibodies in clinically well-defined patients. Diagn Microbiol Infect Dis. 2015 Nov;83(3):222-8. DOI: 10.1016/j.diagmicrobio.2015.07.007
[81] Maraspin V, Cimperman J, Lotric-Furlan S, Ruzić-Sabljić E, Jurca T, Picken RN, Strle F. Solitary borrelial lymphocytoma in adult patients. Wien Klin Wochenschr. 2002 Jul 31;114(13-14):515-23.
[82] Maraspin V, Nahtigal Klevišar M, Ružić-Sabljić E, Lusa L, Strle F. Borrelial Lymphocytoma in Adult Patients. Clin Infect Dis. 2016 Oct 1;63(7):914-21. DOI: 10.1093/cid/ciw417
[83] Hovmark A, Asbrink E, Olsson I. The spirochetal etiology of lymphadenosis benigna cutis solitaria. Acta Derm Venereol. 1986;66(6):479-84.
[84] Lenormand C, Jaulhac B, De Martino S, Barthel C, Lipsker D. Species of Borrelia burgdorferi complex that cause borrelial lymphocytoma in France. Br J Dermatol. 2009 Jul;161(1):174-6. DOI: 10.1111/j.1365-2133.2009.09100.x
[85] Arnež M, Ružić-Sabljić E. Borrelial Lymphocytoma in Children. Pediatr Infect Dis J. 2015 Dec;34(12):1319-22. DOI: 10.1097/INF.0000000000000884
[86] Glatz M, Resinger A, Semmelweis K, Ambros-Rudolph CM, Müllegger RR. Clinical spectrum of skin manifestations of Lyme borreliosis in 204 children in Austria. Acta Derm Venereol. 2015 May;95(5):565-71. DOI: 10.2340/00015555-2000
[87] Arnez M, Pleterski-Rigler D, Luznik-Bufon T, Ruzic-Sabljić E, Strle F. Solitary and multiple erythema migrans in children: comparison of demographic, clinical and laboratory findings. Infection. 2003 Dec;31(6):404-9. DOI: 10.1007/s15010-003-4007-3
[88] Arnež M, Ružić-Sabljić E. Borrelia burgdorferi sensu lato bacteremia in Slovenian children with solitary and multiple erythema migrans. Pediatr Infect Dis J. 2011 Nov;30(11):988-90. DOI: 10.1097/INF.0b013e318225b8c3
[89] Arnez M, Pleterski-Rigler D, Luznik-Bufon T, Ruzić-Sabljić E, Strle F. Children with multiple erythema migrans: are there any pre-treatment symptoms and/or signs suggestive for central nervous system involvement? Wien Klin Wochenschr. 2002 Jul 31;114(13-14):524-9.
[90] Liveris D, Schwartz I, McKenna D, Nowakowski J, Nadelman RB, DeMarco J, Iyer R, Cox ME, Holmgren D, Wormser GP. Quantitation of cell-associated borrelial DNA in the blood of Lyme disease patients with erythema migrans. Eur J Clin Microbiol Infect Dis. 2012 May;31(5):791-5. DOI: 10.1007/s10096-011-1376-x.
[91] Asbrink E. Acrodermatitis chronica atrophicans. Clin Dermatol. 1993 Jul-Sep;11(3):369-75. DOI: 10.1016/0738-081x(93)90092-q
[92] Brzonova I, Wollenberg A, Prinz JC. Acrodermatitis chronica atrophicans affecting all four limbs in an 11-year-old girl. Br J Dermatol. 2002 Aug;147(2):375-8. DOI: 10.1046/j.1365-2133.2002.04792.x
[93] Zalaudek I, Leinweber B, Kerl H, Müllegger RR. Acrodermatitis chronica atrophicans in a 15-year-old girl misdiagnosed as venous insufficiency for 6 years. J Am Acad Dermatol. 2005 Jun;52(6):1091-4. DOI: 10.1016/j.jaad.2005.01.125
[94] Andres C, Ziai M, Bruckbauer H, Ring J, Hofmann H. Acrodermatitis chronica atrophicans in two children. Int J Dermatol. 2010 Feb;49(2):180-3. DOI: 10.1111/j.1365-4632.2009.04194.x
[95] Maraspin V, Mrvič T, Ružić-Sabljić E, Jurčić V, Strle F. Acrodermatitis chronica atrophicans in children: Report on two cases and review of the literature. Ticks Tick Borne Dis. 2019 Jan;10(1):180-5. DOI: 10.1016/j.ttbdis.2018.10.009
[96] Hopf HC. Peripheral neuropathy in acrodermatitis chronica atrophicans (Herxheimer). J Neurol Neurosurg Psychiatry. 1975 May;38(5):452-8. DOI: 10.1136/jnnp.38.5.452
[97] Kristoferitsch W, Sluga E, Graf M, Partsch H, Neumann R, Stanek G, Budka H. Neuropathy associated with acrodermatitis chronica atrophicans. Clinical and morphological features. Ann N Y Acad Sci. 1988;539:35-45. DOI: 10.1111/j.1749-6632.1988.tb31836.x
[98] Kindstrand E, Nilsson BY, Hovmark A, Pirskanen R, Asbrink E. Peripheral neuropathy in acrodermatitis chronica atrophicans - a late Borrelia manifestation. Acta Neurol Scand. 1997 Jun;95(6):338-45. DOI: 10.1111/j.1600-0404.1997.tb00222.x
[99] Asbrink E, Hovmark A. Successful cultivation of spirochetes from skin lesions of patients with erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans. Acta Pathol Microbiol Immunol Scand B. 1985 Apr;93(2):161-3. DOI: 10.1111/j.1699-0463.1985.tb02870.x
[100] Brehmer-Andersson E, Hovmark A, Asbrink E. Acrodermatitis chronica atrophicans: histopathologic findings and clinical correlations in 111 cases. Acta Derm Venereol. 1998 May;78(3):207-13. DOI: 10.1080/000155598441558
[101] Arnez M, Pleterski-Rigler D, Luznik-Bufon T, Ruzić-Sabljić E, Strle F. Solitary erythema migrans in children: comparison of treatment with azithromycin and phenoxymethylpenicillin. Wien Klin Wochenschr. 2002 Jul 31;114(13-14):498-504.
[102] Travaglino A, Varricchio S, Pace M, Russo D, Picardi M, Baldo A, Staibano S, Mascolo M. Borrelia burgdorferi in primary cutaneous lymphomas: a systematic review and meta-analysis. J Dtsch Dermatol Ges. 2020 Dec;18(12):1379-84. DOI: 10.1111/ddg.14289
[103] Hunfeld KP, Wichelhaus TA, Brade V. Borreliose. In: Thomas L, editor. Labor und Diagnose. 8th ed. 2012. p. 1955-65.
[104] Lohr B, Fingerle V, Norris DE, Hunfeld KP. Laboratory diagnosis of Lyme borreliosis: Current state of the art and future perspectives. Crit Rev Clin Lab Sci. 2018 Jun;55(4):219-45. DOI: 10.1080/10408363.2018.1450353
[105] Hunfeld KP, Stanek G, Straube E, Hagedorn HJ, Schörner C, Mühlschlegel F, Brade V. Quality of Lyme disease serology. Lessons from the German Proficiency Testing Program 1999-2001. A preliminary report. Wien Klin Wochenschr. 2002 Jul 31;114(13-14):591-600.
[106] Fingerle V, Eiffert H, Gessner A, Göbel U, Hofmann H, Hunfeld KP, et al. MiQ 12: Lyme Borreliose. In: Podbielski A, Abele-Horn M, Hermann M, Kniehl E, Mauch H, Rüssmann H, editors. MiQ - Qualitätsstandars in der mikrobiologisch-infektiologischen Untersuchung. 2nd ed. München, Jena: Elsevier, Urban & Fischer; 2017.
[107] Münchhoff P, Wilske B, Preac-Mursic V, Schierz G. Antibodies against Borrelia burgdorferi in Bavarian forest workers. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Feb;263(3):412-9. DOI: 10.1016/s0176-6724(87)80101-3
[108] Cetin E, Sotoudeh M, Auer H, Stanek G. Paradigm Burgenland: risk of Borrelia burgdorferi sensu lato infection indicated by variable seroprevalence rates in hunters. Wien Klin Wochenschr. 2006 Nov;118(21-22):677-81. DOI: 10.1007/s00508-006-0694-y
[109] Dehnert M, Fingerle V, Klier C, Talaska T, Schlaud M, Krause G, Wilking H, Poggensee G. Seropositivity of Lyme borreliosis and associated risk factors: a population-based study in Children and Adolescents in Germany (KiGGS). PLoS One. 2012;7(8):e41321. DOI: 10.1371/journal.pone.0041321
[110] Nyman D, Willén L, Jansson C, Carlsson SA, Granlund H, Wahlberg P. VlsE C6 peptide and IgG ELISA antibody analysis for clinical diagnosis of Lyme borreliosis in an endemic area. Clin Microbiol Infect. 2006 May;12(5):496-7. DOI: 10.1111/j.1469-0691.2006.01374.x
[111] Branda JA, Strle F, Strle K, Sikand N, Ferraro MJ, Steere AC. Performance of United States serologic assays in the diagnosis of Lyme borreliosis acquired in Europe. Clin Infect Dis. 2013 Aug;57(3):333-40. DOI: 10.1093/cid/cit235
[112] Hunfeld KP, Kraiczy P. When is the best time to order a Western blot and how should it be interpreted? Curr Probl Dermatol. 2009;37:167-77. DOI: 10.1159/000213074
[113] Hauser U, Lehnert G, Wilske B. Validity of interpretation criteria for standardized Western blots (immunoblots) for serodiagnosis of Lyme borreliosis based on sera collected throughout Europe. J Clin Microbiol. 1999 Jul;37(7):2241-7. DOI: 10.1128/JCM.37.7.2241-2247.1999
[114] Goettner G, Schulte-Spechtel U, Hillermann R, Liegl G, Wilske B, Fingerle V. Improvement of Lyme borreliosis serodiagnosis by a newly developed recombinant immunoglobulin G (IgG) and IgM line immunoblot assay and addition of VlsE and DbpA homologues. J Clin Microbiol. 2005 Aug;43(8):3602-9. DOI: 10.1128/JCM.43.8.3602-3609.2005
[115] Branda JA, Steere AC. Laboratory Diagnosis of Lyme Borreliosis. Clin Microbiol Rev. 2021 Jan 27;34(2):e00018-19. DOI: 10.1128/CMR.00018-19
[116] Lipsett SC, Branda JA, Nigrovic LE. Evaluation of the Modified Two-Tiered Testing Method for Diagnosis of Lyme Disease in Children. J Clin Microbiol. 2019 Sep 24;57(10):e00547-19. DOI: 10.1128/JCM.00547-19
[117] Maulden AB, Garro AC, Balamuth F, Levas MN, Bennett JE, Neville DN, Branda JA, Nigrovic LE. Two-Tier Lyme Disease Serology Test Results Can Vary According to the Specific First-Tier Test Used. J Pediatric Infect Dis Soc. 2020 Apr 30;9(2):128-33. DOI: 10.1093/jpids/piy133
[118] Bunikis J, Barbour AG. Laboratory testing for suspected Lyme disease. Med Clin North Am. 2002 Mar;86(2):311-40. DOI: 10.1016/s0025-7125(03)00089-0
[119] Reischl U, Drosten C, Geißdörfer W, Göbel U, Hoffmann KS, Mauch H, et al. MiQ 1: Nukleinsäure-Amplifikationstechniken (NAT). In: Podbielski A, Abele-Horn M, Hermann M, Kniehl E, Mauch H, Rüssmann H, editors. MiQ - Qualitätsstandars in der mikrobiologisch-infektiologischen Untersuchung. 3rd ed. München, Jena: Urban & Fischer; 2011.
[120] Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521-5.
[121] Preac-Mursic V, Wilske B, Schierz G. European Borrelia burgdorferi isolated from humans and ticks culture conditions and antibiotic susceptibility. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):112-8. DOI: 10.1016/s0176-6724(86)80110-9
[122] Stanek G, Klein J, Bittner R, Glogar D. Borrelia burgdorferi as an etiologic agent in chronic heart failure? Scand J Infect Dis Suppl. 1991;77:85-7.
[123] Preac-Mursic V, Pfister HW, Spiegel H, Burk R, Wilske B, Reinhardt S, Böhmer R. First isolation of Borrelia burgdorferi from an iris biopsy. J Clin Neuroophthalmol. 1993 Sep;13(3):155-61; discussion 162.
[124] van Dam AP, Kuiper H, Vos K, Widjojokusumo A, de Jongh BM, Spanjaard L, Ramselaar AC, Kramer MD, Dankert J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993 Oct;17(4):708-17. DOI: 10.1093/clinids/17.4.708
[125] Kuiper H, Cairo I, Van Dam A, De Jongh B, Ramselaar T, Spanjaard L, Dankert J. Solitary erythema migrans: a clinical, laboratory and epidemiological study of 77 Dutch patients. Br J Dermatol. 1994 Apr;130(4):466-72. DOI: 10.1111/j.1365-2133.1994.tb03379.x
[126] Picken RN, Strle F, Picken MM, Ruzic-Sabljic E, Maraspin V, Lotric-Furlan S, Cimperman J. Identification of three species of Borrelia burgdorferi sensu lato (B. burgdorferi sensu stricto, B. garinii, and B. afzelii) among isolates from acrodermatitis chronica atrophicans lesions. J Invest Dermatol. 1998 Mar;110(3):211-4. DOI: 10.1046/j.1523-1747.1998.00130.x
[127] Cerar T, Ruzić-Sabljić E, Glinsek U, Zore A, Strle F. Comparison of PCR methods and culture for the detection of Borrelia spp. in patients with erythema migrans. Clin Microbiol Infect. 2008 Jul;14(7):653-8. DOI: 10.1111/j.1469-0691.2008.02013.x
[128] Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of lyme borreliosis. Clin Microbiol Rev. 2005 Jul;18(3):484-509. DOI: 10.1128/CMR.18.3.484-509.2005
[129] van Dam AP. Molecular diagnosis of Borrelia bacteria for the diagnosis of Lyme disease. Expert Opin Med Diagn. 2011 Mar;5(2):135-49. DOI: 10.1517/17530059.2011.555396
[130] Moter SE, Hofmann H, Wallich R, Simon MM, Kramer MD. Detection of Borrelia burgdorferi sensu lato in lesional skin of patients with erythema migrans and acrodermatitis chronica atrophicans by ospA-specific PCR. J Clin Microbiol. 1994 Dec;32(12):2980-8. DOI: 10.1128/jcm.32.12.2980-2988.1994
[131] von Stedingk LV, Olsson I, Hanson HS, Asbrink E, Hovmark A. Polymerase chain reaction for detection of Borrelia burgdorferi DNA in skin lesions of early and late Lyme borreliosis. Eur J Clin Microbiol Infect Dis. 1995 Jan;14(1):1-5. DOI: 10.1007/BF02112610
[132] Muellegger R, Zoechling N, Schluepen EM, Soyer HP, Hoedl S, Kerl, Volkenandt M. Polymerase chain reaction control of antibiotic treatment in dermatoborreliosis. Infection. 1996 Jan-Feb;24(1):76-9. DOI: 10.1007/BF01780664
[133] Brettschneider S, Bruckbauer H, Klugbauer N, Hofmann H. Diagnostic value of PCR for detection of Borrelia burgdorferi in skin biopsy and urine samples from patients with skin borreliosis. J Clin Microbiol. 1998 Sep;36(9):2658-65. DOI: 10.1128/JCM.36.9.2658-2665.1998
[134] Bockenstedt LK, Gonzalez DG, Haberman AM, Belperron AA. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J Clin Invest. 2012 Jul;122(7):2652-60. DOI: 10.1172/JCI58813
[135] Iyer R, Mukherjee P, Wang K, Simons J, Wormser GP, Schwartz I. Detection of Borrelia burgdorferi nucleic acids after antibiotic treatment does not confirm viability. J Clin Microbiol. 2013 Mar;51(3):857-62. DOI: 10.1128/JCM.02785-12
[136] Pícha D, Moravcová L, Vaňousová D, Hercogová J, Blechová Z. DNA persistence after treatment of Lyme borreliosis. Folia Microbiol (Praha). 2014 Mar;59(2):115-25. DOI: 10.1007/s12223-013-0272-4
[137] Rauter C, Mueller M, Diterich I, Zeller S, Hassler D, Meergans T, Hartung T. Critical evaluation of urine-based PCR assay for diagnosis of Lyme borreliosis. Clin Diagn Lab Immunol. 2005 Aug;12(8):910-7. DOI: 10.1128/CDLI.12.8.910-917.2005
[138] Aberer E, Bergmann AR, Derler AM, Schmidt B. Course of Borrelia burgdorferi DNA shedding in urine after treatment. Acta Derm Venereol. 2007;87(1):39-42. DOI: 10.2340/00015555-0172
[139] Colli C, Leinweber B, Müllegger R, Chott A, Kerl H, Cerroni L. Borrelia burgdorferi-associated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004 Mar;31(3):232-40. DOI: 10.1111/j.0303-6987.2003.00167.x
[140] Raffetin A, Saunier A, Bouiller K, Caraux-Paz P, Eldin C, Gallien S, Jouenne R, Belkacem A, Salomon J, Patey O, Talagrand-Reboul E, Jaulhac B, Grillon A. Unconventional diagnostic tests for Lyme borreliosis: a systematic review. Clin Microbiol Infect. 2020 Jan;26(1):51-9. DOI: 10.1016/j.cmi.2019.06.033
[141] Baarsma ME, van de Schoor FR, Gauw SA, Vrijmoeth HD, Ursinus J, Goudriaan N, Popa CD, Ter Hofstede HJ, Leeflang MM, Kremer K, van den Wijngaard CC, Kullberg BJ, Joosten LA, Hovius JW. Diagnostic parameters of cellular tests for Lyme borreliosis in Europe (VICTORY study): a case-control study. Lancet Infect Dis. 2022 Sep;22(9):1388-96. DOI: 10.1016/S1473-3099(22)00205-5
[142] Torbahn G, Hofmann H, Allert R, Freitag MH, Dersch R, Fingerle V, Sommer H, Motschall E, Meerpohl JJ, Schmucker C. Efficacy and safety of pharmacological agents in the treatment of erythema migrans in early Lyme borreliosis-systematic review protocol. Syst Rev. 2016 May 3;5:73. DOI: 10.1186/s13643-016-0251-3
[143] Dattwyler RJ, Volkman DJ, Conaty SM, Platkin SP, Luft BJ. Amoxycillin plus probenecid versus doxycycline for treatment of erythema migrans borreliosis. Lancet. 1990 Dec 8;336(8728):1404-6. DOI: 10.1016/0140-6736(90)93103-v
[144] Hunfeld KP, Rödel R, Wichelhaus TA. In vitro activity of eight oral cephalosporins against Borrelia burgdorferi. Int J Antimicrob Agents. 2003 Apr;21(4):313-8. DOI: 10.1016/s0924-8579(03)00005-0
[145] Dattwyler RJ, Luft BJ, Kunkel MJ, Finkel MF, Wormser GP, Rush TJ, Grunwaldt E, Agger WA, Franklin M, Oswald D, Cockey L, Maladorno D. Ceftriaxone compared with doxycycline for the treatment of acute disseminated Lyme disease. N Engl J Med. 1997 Jul 31;337(5):289-94. DOI: 10.1056/NEJM199707313370501
[146] Weber K, Wilske B, Preac-Mursic V, Thurmayr R. Azithromycin versus penicillin V for the treatment of early Lyme borreliosis. Infection. 1993 Nov-Dec;21(6):367-72. DOI: 10.1007/BF01728915
[147] Luft BJ, Dattwyler RJ, Johnson RC, Luger SW, Bosler EM, Rahn DW, Masters EJ, Grunwaldt E, Gadgil SD. Azithromycin compared with amoxicillin in the treatment of erythema migrans. A double-blind, randomized, controlled trial. Ann Intern Med. 1996 May 1;124(9):785-91. DOI: 10.7326/0003-4819-124-9-199605010-00002
[148] Barsic B, Maretic T, Majerus L, Strugar J. Comparison of azithromycin and doxycycline in the treatment of erythema migrans. Infection. 2000 May-Jun;28(3):153-6. DOI: 10.1007/s150100050069
[149] Nizič T, Velikanje E, Ružić-Sabljić E, Arnež M. Solitary erythema migrans in children: comparison of treatment with clarithromycin and amoxicillin. Wien Klin Wochenschr. 2012 Jul;124(13-14):427-33. DOI: 10.1007/s00508-012-0194-1
[150] Hansen K, Hovmark A, Lebech AM, Lebech K, Olsson I, Halkier-Sørensen L, Olsson E, Asbrink E. Roxithromycin in Lyme borreliosis: discrepant results of an in vitro and in vivo animal susceptibility study and a clinical trial in patients with erythema migrans. Acta Derm Venereol. 1992 Aug;72(4):297-300.
[151] Bennet L, Danell S, Berglund J. Clinical outcome of erythema migrans after treatment with phenoxymethyl penicillin. Scand J Infect Dis. 2003;35(2):129-31. DOI: 10.1080/0036554021000027009
[152] Aberer E, Kahofer P, Binder B, Kinaciyan T, Schauperl H, Berghold A. Comparison of a two- or three-week regimen and a review of treatment of erythema migrans with phenoxymethylpenicillin. Dermatology. 2006;212(2):160-7. DOI: 10.1159/000090657
[153] Arnez M. Antibiotic treatment of children with erythema migrans. Clin Infect Dis. 2007 Apr 15;44(8):1133-4; author reply 1137-9. DOI: 10.1086/512978
[154] Aberer E, Breier F, Stanek G, Schmidt B. Success and failure in the treatment of acrodermatitis chronica atrophicans. Infection. 1996 Jan-Feb;24(1):85-7. DOI: 10.1007/BF01780666
[155] Stupica D, Lusa L, Ruzić-Sabljić E, Cerar T, Strle F. Treatment of erythema migrans with doxycycline for 10 days versus 15 days. Clin Infect Dis. 2012 Aug;55(3):343-50. DOI: 10.1093/cid/cis402
[156] Cerar D, Cerar T, Ruzić-Sabljić E, Wormser GP, Strle F. Subjective symptoms after treatment of early Lyme disease. Am J Med. 2010 Jan;123(1):79-86. DOI: 10.1016/j.amjmed.2009.05.011
[157] Kowalski TJ, Tata S, Berth W, Mathiason MA, Agger WA. Antibiotic treatment duration and long-term outcomes of patients with early lyme disease from a lyme disease-hyperendemic area. Clin Infect Dis. 2010 Feb 15;50(4):512-20. DOI: 10.1086/649920
[158] Weber K. Treatment failure in erythema migrans--a review. Infection. 1996 Jan-Feb;24(1):73-5. DOI: 10.1007/BF01780663
[159] Schmidli J, Hunziker T, Moesli P, Schaad UB. Cultivation of Borrelia burgdorferi from joint fluid three months after treatment of facial palsy due to Lyme borreliosis. J Infect Dis. 1988 Oct;158(4):905-6. DOI: 10.1093/infdis/158.4.905
[160] Häupl T, Hahn G, Rittig M, Krause A, Schoerner C, Schönherr U, Kalden JR, Burmester GR. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum. 1993 Nov;36(11):1621-6. DOI: 10.1002/art.1780361118
[161] Priem S, Burmester GR, Kamradt T, Wolbart K, Rittig MG, Krause A. Detection of Borrelia burgdorferi by polymerase chain reaction in synovial membrane, but not in synovial fluid from patients with persisting Lyme arthritis after antibiotic therapy. Ann Rheum Dis. 1998 Feb;57(2):118-21. DOI: 10.1136/ard.57.2.118
[162] Breier F, Khanakah G, Stanek G, Kunz G, Aberer E, Schmidt B, Tappeiner G. Isolation and polymerase chain reaction typing of Borrelia afzelii from a skin lesion in a seronegative patient with generalized ulcerating bullous lichen sclerosus et atrophicus. Br J Dermatol. 2001 Feb;144(2):387-92. DOI: 10.1046/j.1365-2133.2001.04034.x
[163] Nadelman RB, Hanincová K, Mukherjee P, Liveris D, Nowakowski J, McKenna D, Brisson D, Cooper D, Bittker S, Madison G, Holmgren D, Schwartz I, Wormser GP. Differentiation of reinfection from relapse in recurrent Lyme disease. N Engl J Med. 2012 Nov 15;367(20):1883-90. DOI: 10.1056/NEJMoa1114362
[164] Jares TM, Mathiason MA, Kowalski TJ. Functional outcomes in patients with Borrelia burgdorferi reinfection. Ticks Tick Borne Dis. 2014 Feb;5(1):58-62. DOI: 10.1016/j.ttbdis.2013.09.002
[165] Maraspin V, Bogovič P, Rojko T, Ogrinc K, Ružić-Sabljić E, Strle F. Early Lyme Borreliosis in Patients Treated with Tumour Necrosis Factor-Alfa Inhibitors. J Clin Med. 2019 Nov 2;8(11):1857. DOI: 10.3390/jcm8111857
[166] Hunfeld KP, Ruzic-Sabljic E, Norris DE, Kraiczy P, Strle F. In vitro susceptibility testing of Borrelia burgdorferi sensu lato isolates cultured from patients with erythema migrans before and after antimicrobial chemotherapy. Antimicrob Agents Chemother. 2005 Apr;49(4):1294-301. DOI: 10.1128/AAC.49.4.1294-1301.2005
[167] Ruzić-Sabljić E, Podreka T, Maraspin V, Strle F. Susceptibility of Borrelia afzelii strains to antimicrobial agents. Int J Antimicrob Agents. 2005 Jun;25(6):474-8. DOI: 10.1016/j.ijantimicag.2005.02.007
[168] Hunfeld KP, Brade V. Antimicrobial susceptibility of Borrelia burgdorferi sensu lato: what we know, what we don't know, and what we need to know. Wien Klin Wochenschr. 2006 Nov;118(21-22):659-68. DOI: 10.1007/s00508-006-0693-z
[169] Morgenstern K, Baljer G, Norris DE, Kraiczy P, Hanssen-Hübner C, Hunfeld KP. In vitro susceptibility of Borrelia spielmanii to antimicrobial agents commonly used for treatment of Lyme disease. Antimicrob Agents Chemother. 2009 Mar;53(3):1281-4. DOI: 10.1128/AAC.01247-08
[170] Klempner MS, Hu LT, Evans J, Schmid CH, Johnson GM, Trevino RP, Norton D, Levy L, Wall D, McCall J, Kosinski M, Weinstein A. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001 Jul 12;345(2):85-92. DOI: 10.1056/NEJM200107123450202
[171] Kaplan RF, Trevino RP, Johnson GM, Levy L, Dornbush R, Hu LT, Evans J, Weinstein A, Schmid CH, Klempner MS. Cognitive function in post-treatment Lyme disease: do additional antibiotics help? Neurology. 2003 Jun 24;60(12):1916-22. DOI: 10.1212/01.wnl.0000068030.26992.25
[172] Krupp LB, Hyman LG, Grimson R, Coyle PK, Melville P, Ahnn S, Dattwyler R, Chandler B. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology. 2003 Jun 24;60(12):1923-30. DOI: 10.1212/01.wnl.0000071227.23769.9e
[173] Feder HM Jr, Johnson BJ, O'Connell S, Shapiro ED, Steere AC, Wormser GP; Ad Hoc International Lyme Disease Group; Agger WA, Artsob H, Auwaerter P, Dumler JS, Bakken JS, Bockenstedt LK, Green J, Dattwyler RJ, Munoz J, Nadelman RB, Schwartz I, Draper T, McSweegan E, Halperin JJ, Klempner MS, Krause PJ, Mead P, Morshed M, Porwancher R, Radolf JD, Smith RP Jr, Sood S, Weinstein A, Wong SJ, Zemel L. A critical appraisal of "chronic Lyme disease". N Engl J Med. 2007 Oct 4;357(14):1422-30. DOI: 10.1056/NEJMra072023
[174] Fallon BA, Keilp JG, Corbera KM, Petkova E, Britton CB, Dwyer E, Slavov I, Cheng J, Dobkin J, Nelson DR, Sackeim HA. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. 2008 Mar 25;70(13):992-1003. DOI: 10.1212/01.WNL.0000284604.61160.2d
[175] Klempner MS, Baker PJ, Shapiro ED, Marques A, Dattwyler RJ, Halperin JJ, Wormser GP. Treatment trials for post-Lyme disease symptoms revisited. Am J Med. 2013 Aug;126(8):665-9. DOI: 10.1016/j.amjmed.2013.02.014
[176] Berende A, ter Hofstede HJ, Vos FJ, van Middendorp H, Vogelaar ML, Tromp M, van den Hoogen FH, Donders AR, Evers AW, Kullberg BJ. Randomized Trial of Longer-Term Therapy for Symptoms Attributed to Lyme Disease. N Engl J Med. 2016 Mar 31;374(13):1209-20. DOI: 10.1056/NEJMoa1505425
[177] Berende A, ter Hofstede HJ, Donders AR, van Middendorp H, Kessels RP, Adang EM, Vos FJ, Evers AW, Kullberg BJ. Persistent Lyme Empiric Antibiotic Study Europe (PLEASE)--design of a randomized controlled trial of prolonged antibiotic treatment in patients with persistent symptoms attributed to Lyme borreliosis. BMC Infect Dis. 2014 Oct 16;14:543. DOI: 10.1186/s12879-014-0543-y
[178] Lakos A, Solymosi N. Maternal Lyme borreliosis and pregnancy outcome. Int J Infect Dis. 2010 Jun;14(6):e494-8. DOI: 10.1016/j.ijid.2009.07.019
[179] Maraspin V, Ružić-Sabljić E, Pleterski-Rigler D, Strle F. Pregnant women with erythema migrans and isolation of borreliae from blood: course and outcome after treatment with ceftriaxone. Diagn Microbiol Infect Dis. 2011 Dec;71(4):446-8. DOI: 10.1016/j.diagmicrobio.2011.07.017
[180] Buonomo A, Nucera E, Pecora V, Rizzi A, Aruanno A, Pascolini L, Ricci AG, Colagiovanni A, Schiavino D. Cross-reactivity and tolerability of cephalosporins in patients with cell-mediated allergy to penicillins. J Investig Allergol Clin Immunol. 2014;24(5):331-7.
[181] Wormser GP, Strle F, Shapiro ED. Is Doxycycline Appropriate for Routine Treatment of Young Children With Erythema Migrans? Pediatr Infect Dis J. 2019 Nov;38(11):1113-4. DOI: 10.1097/INF.0000000000002453
[182] Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006 Nov 1;43(9):1089-134. DOI: 10.1086/508667
[183] Kullberg BJ, Vrijmoeth HD, van de Schoor F, Hovius JW. Lyme borreliosis: diagnosis and management. BMJ. 2020 May 26;369:m1041. DOI: 10.1136/bmj.m1041
[184] Puéchal X, Sibilia J. What should be done in case of persistent symptoms after adequate antibiotic treatment for Lyme disease? Curr Probl Dermatol. 2009;37:191-9. DOI: 10.1159/000213077
[185] Deutsche Gesellschaft für Neurologie e.V. (DGN). S2k-Leitlinie Diagnose und nicht interventionelle Therapie neuropathischer Schmerzen. Version 4.3. AWMF-Registernummer 030-114. Berlin: AWMF; 2019. Available from: https://register.awmf.org/de/leitlinien/detail/030-114
[186] Deutsche Gesellschaft für Psychosomatische Medizin und Ärztliche Psychotherapie e.V. (DGPM); Deutsches Kollegium für Psychosomatische Medizin e.V. (DKPM). S3-Leitlinie Funktionelle Körperbeschwerden. Version 2.0. AWMF-Registernummer 051-001. Berlin: AWMF; 2018. Available from: https://register.awmf.org/de/leitlinien/detail/051-001
[187] Nemeth J, Bernasconi E, Heininger U, Abbas M, Nadal D, Strahm C, Erb S, Zimmerli S, Furrer H, Delaloye J, Kuntzer T, Altpeter E, Sturzenegger M, Weber R; Swiss Society For Infectious Diseases; Swiss Society For Neurology. Update of the Swiss guidelines on post-treatment Lyme disease syndrome. Swiss Med Wkly. 2016 Dec 5;146:w14353. DOI: 10.4414/smw.2016.14353
[188] Lupi E, Hatz C, Schlagenhauf P. The efficacy of repellents against Aedes, Anopheles, Culex and Ixodes spp. - a literature review. Travel Med Infect Dis. 2013 Nov-Dec;11(6):374-411. DOI: 10.1016/j.tmaid.2013.10.005
[189] Faulde MK, Rutenfranz M, Keth A, Hepke J, Rogge M, Görner A. Pilot study assessing the effectiveness of factory-treated, long-lasting permethrin-impregnated clothing for the prevention of tick bites during occupational tick exposure in highly infested military training areas, Germany. Parasitol Res. 2015 Feb;114(2):671-8. DOI: 10.1007/s00436-014-4232-y
[190] Pages F, Dautel H, Duvallet G, Kahl O, de Gentile L, Boulanger N. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014 Feb;14(2):85-93. DOI: 10.1089/vbz.2013.1410
[191] Leenders AC. Single-dose doxycycline for the prevention of Lyme disease. N Engl J Med. 2001 Nov 1;345(18):1349; author reply 1349-50.
[192] Nadelman RB, Nowakowski J, Fish D, Falco RC, Freeman K, McKenna D, Welch P, Marcus R, Agüero-Rosenfeld ME, Dennis DT, Wormser GP; Tick Bite Study Group. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001 Jul 12;345(2):79-84. DOI: 10.1056/NEJM200107123450201
[193] Harms MG, Hofhuis A, Sprong H, Bennema SC, Ferreira JA, Fonville M, Docters van Leeuwen A, Assendelft WJJ, Van Weert HCPM, Van Pelt W, Van den Wijngaard CC. A single dose of doxycycline after an ixodes ricinus tick bite to prevent Lyme borreliosis: An open-label randomized controlled trial. J Infect. 2021 Jan;82(1):98-104. DOI: 10.1016/j.jinf.2020.06.032
[194] Wilske B. Epidemiology and diagnosis of Lyme borreliosis. Ann Med. 2005;37(8):568-79. DOI: 10.1080/07853890500431934
[195] Knauer J, Krupka I, Fueldner C, Lehmann J, Straubinger RK. Evaluation of the preventive capacities of a topically applied azithromycin formulation against Lyme borreliosis in a murine model. J Antimicrob Chemother. 2011 Dec;66(12):2814-22. DOI: 10.1093/jac/dkr371
[196] Piesman J, Hojgaard A, Ullmann AJ, Dolan MC. Efficacy of an experimental azithromycin cream for prophylaxis of tick-transmitted lyme disease spirochete infection in a murine model. Antimicrob Agents Chemother. 2014;58(1):348-51. DOI: 10.1128/AAC.01932-13
[197] Schwameis M, Kündig T, Huber G, von Bidder L, Meinel L, Weisser R, Aberer E, Härter G, Weinke T, Jelinek T, Fätkenheuer G, Wollina U, Burchard GD, Aschoff R, Nischik R, Sattler G, Popp G, Lotte W, Wiechert D, Eder G, Maus O, Staubach-Renz P, Gräfe A, Geigenberger V, Naudts I, Sebastian M, Reider N, Weber R, Heckmann M, Reisinger EC, Klein G, Wantzen J, Jilma B. Topical azithromycin for the prevention of Lyme borreliosis: a randomised, placebo-controlled, phase 3 efficacy trial. Lancet Infect Dis. 2017 Mar;17(3):322-9. DOI: 10.1016/S1473-3099(16)30529-1
[198] Wallich R, Kramer MD, Simon MM. The recombinant outer surface protein A (lipOspA) of Borrelia burgdorferi: a Lyme disease vaccine. Infection. 1996 Sep-Oct;24(5):396-7. DOI: 10.1007/BF01716093
[199] Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, Nowakowski J, Schmid CH, Laukamp S, Buscarino C, Krause DS. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. N Engl J Med. 1998 Jul 23;339(4):209-15. DOI: 10.1056/NEJM199807233390401
[200] Kalish RS, Wood JA, Golde W, Bernard R, Davis LE, Grimson RC, Coyle PK, Luft BJ. Human T lymphocyte response to Borrelia burgdorferi infection: no correlation between human leukocyte function antigen type 1 peptide response and clinical status. J Infect Dis. 2003 Jan 1;187(1):102-8. DOI: 10.1086/346059
[201] Abbott A. Lyme disease: uphill struggle. Nature. 2006 Feb 2;439(7076):524-5. DOI: 10.1038/439524a
[202] Nigrovic LE, Thompson KM. The Lyme vaccine: a cautionary tale. Epidemiol Infect. 2007 Jan;135(1):1-8. DOI: 10.1017/S0950268806007096
[203] Barrett PN, Portsmouth D. A novel multivalent OspA vaccine against Lyme borreliosis shows promise in Phase I/II studies. Expert Rev Vaccines. 2013 Sep;12(9):973-5. DOI: 10.1586/14760584.2013.824704
[204] Deutsche Dermatologische Gesellschaft e.V. (DDG). S2k-Leitlinie Kutane Lyme Borreliose. Version 3.0. AWMF-Registernummer 013-044. Berlin: AWMF; 2023. Available from: https://register.awmf.org/de/leitlinien/detail/013-044
[205] Huppertz HI, Bartmann P, Heininger U, Fingerle V, Kinet M, Klein R, Korenke GC, Nentwich HJ; Committee for Infectious Diseases and Vaccinations of the German Academy for Pediatrics and Adolescent Health. Rational diagnostic strategies for Lyme borreliosis in children and adolescents: recommendations by the Committee for Infectious Diseases and Vaccinations of the German Academy for Pediatrics and Adolescent Health. Eur J Pediatr. 2012 Nov;171(11):1619-24. DOI: 10.1007/s00431-012-1779-4
[206] Mygland A, Ljøstad U, Fingerle V, Rupprecht T, Schmutzhard E, Steiner I; European Federation of Neurological Societies. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol. 2010 Jan;17(1):8-16, e1-4. DOI: 10.1111/j.1468-1331.2009.02862.x
[207] Brouqui P, Bacellar F, Baranton G, Birtles RJ, Bjoërsdorff A, Blanco JR, Caruso G, Cinco M, Fournier PE, Francavilla E, Jensenius M, Kazar J, Laferl H, Lakos A, Lotric Furlan S, Maurin M, Oteo JA, Parola P, Perez-Eid C, Peter O, Postic D, Raoult D, Tellez A, Tselentis Y, Wilske B; ESCMID Study Group on Coxiella, Anaplasma, Rickettsia and Bartonella; European Network for Surveillance of Tick-Borne Diseases. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin Microbiol Infect. 2004 Dec;10(12):1108-32. DOI: 10.1111/j.1469-0691.2004.01019.x
[208] Canadian Public Health Laboratory Network. The laboratory diagnosis of Lyme borreliosis: Guidelines from the Canadian Public Health Laboratory Network. Can J Infect Dis Med Microbiol. 2007 Mar;18(2):145-8. DOI: 10.1155/2007/495108
[209] Evison J, Aebi C, Francioli P, Péter O, Bassetti S, Gervaix A, Zimmerli S, Weber R. Borretiose de Lyme 2e partie: clinique et traitement [Lyme disease Part 2: clinic and treatment]. Rev Med Suisse. 2006 Apr 5;2(60):925-8, 930-4.
[210] Evison J, Aebi C, Francioli P, Péter O, Bassetti S, Gervaix A, Zimmerli S, Weber R. Borréliose de Lyme 1ère partie: épidémiotogie et diagnostic [Lyme disease Part I: epidemiology and diagnosis]. Rev Med Suisse. 2006 Apr 5;2(60):919-24.
[211] Evison J, Aebi C, Francioli P, Péter O, Bassetti S, Gervaix A, Zimmerli S, Weber R. Borréliose de Lyme 3e partie: prévention, grossesse, états d'immunodéficience, syndrome post-borréliose de Lyme [Lyme disease Part 3: prevention, pregnancy, immunodeficient state, post-Lyme disease syndrome]. Rev Med Suisse. 2006 Apr 5;2(60):935-6, 938-40.
[212] Gocko X, Lenormand C, Lemogne C, Bouiller K, Gehanno JF, Rabaud C, Perrot S, Eldin C, de Broucker T, Roblot F, Toubiana J, Sellal F, Vuillemet F, Sordet C, Fantin B, Lina G, Sobas C, Jaulhac B, Figoni J, Chirouze C, Hansmann Y, Hentgen V, Caumes E, Dieudonné M, Picone O, Bodaghi B, Gangneux JP, Degeilh B, Partouche H, Saunier A, Sotto A, Raffetin A, Monsuez JJ, Michel C, Boulanger N, Cathebras P, Tattevin P; endorsed by the following scientific societies. Lyme borreliosis and other tick-borne diseases. Guidelines from the French scientific societies. Med Mal Infect. 2019 Aug;49(5):296-317. DOI: 10.1016/j.medmal.2019.05.006
[213] Jaulhac B, Saunier A, Caumes E, Bouiller K, Gehanno JF, Rabaud C, Perrot S, Eldin C, de Broucker T, Roblot F, Toubiana J, Sellal F, Vuillemet F, Sordet C, Fantin B, Lina G, Sobas C, Gocko X, Figoni J, Chirouze C, Hansmann Y, Hentgen V, Cathebras P, Dieudonné M, Picone O, Bodaghi B, Gangneux JP, Degeilh B, Partouche H, Lenormand C, Sotto A, Raffetin A, Monsuez JJ, Michel C, Boulanger N, Lemogne C, Tattevin P; endorsed by scientific societies. Lyme borreliosis and other tick-borne diseases. Guidelines from the French scientific societies (II). Biological diagnosis, treatment, persistent symptoms after documented or suspected Lyme borreliosis. Med Mal Infect. 2019 Aug;49(5):335-46. DOI: 10.1016/j.medmal.2019.05.001
[214] Figoni J, Chirouze C, Hansmann Y, Lemogne C, Hentgen V, Saunier A, Bouiller K, Gehanno JF, Rabaud C, Perrot S, Caumes E, Eldin C, de Broucker T, Jaulhac B, Roblot F, Toubiana J, Sellal F, Vuillemet F, Sordet C, Fantin B, Lina G, Gocko X, Dieudonné M, Picone O, Bodaghi B, Gangneux JP, Degeilh B, Partouche H, Lenormand C, Sotto A, Raffetin A, Monsuez JJ, Michel C, Boulanger N, Cathebras P, Tattevin P; endorsed by scientific societies. Lyme borreliosis and other tick-borne diseases. Guidelines from the French Scientific Societies (I): prevention, epidemiology, diagnosis. Med Mal Infect. 2019 Aug;49(5):318-34. DOI: 10.1016/j.medmal.2019.04.381
[215] Lantos PM, Rumbaugh J, Bockenstedt LK, Falck-Ytter YT, Aguero-Rosenfeld ME, Auwaerter PG, Baldwin K, Bannuru RR, Belani KK, Bowie WR, Branda JA, Clifford DB, DiMario FJ, Halperin JJ, Krause PJ, Lavergne V, Liang MH, Meissner HC, Nigrovic LE, Nocton JJJ, Osani MC, Pruitt AA, Rips J, Rosenfeld LE, Savoy ML, Sood SK, Steere AC, Strle F, Sundel R, Tsao J, Vaysbrot EE, Wormser GP, Zemel LS. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the Prevention, Diagnosis and Treatment of Lyme Disease. Clin Infect Dis. 2021 Jan 23;72(1):e1-e48. DOI: 10.1093/cid/ciaa1215
[216] Cameron DJ, Johnson LB, Maloney EL. Evidence assessments and guideline recommendations in Lyme disease: the clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev Anti Infect Ther. 2014 Sep;12(9):1103-35. DOI: 10.1586/14787210.2014.940900
[217] Hofmann H, Aberer E, Bruckbauer H, Fingerle V, Mempel M, Müllegger RR, et al. Kutane Manifestationen der Lyme Borreliose. Berlin: AWMF; 2009.
[218] Pancewicz SA, Garlicki AM, Moniuszko-Malinowska A, Zajkowska J, Kondrusik M, Grygorczuk S, Czupryna P, Dunaj J; Polish Society of Epidemiology and Infectious Diseases. Diagnosis and treatment of tick-borne diseases recommendations of the Polish Society of Epidemiology and Infectious Diseases. Przegl Epidemiol. 2015;69(2):309-16, 421-8.
[219] Gaubitz M, Dressler F, Huppertz HI, Krause A; Kommission Pharmakotherapie der DGRh. Diagnostik und Therapie der Lyme-Arthritis. Empfehlungen der Kommission Pharmakotherapie der DGRh [Diagnosis and treatment of Lyme arthritis. Recommendations of the Pharmacotherapy Commission of the Deutsche Gesellschaft für Rheumatologie (German Society for Rheumatology)]. Z Rheumatol. 2014 Jun;73(5):469-74. DOI: 10.1007/s00393-014-1370-7
Attachments
| Attachment 1 | Annexes (000348_Attachment1.pdf, application/pdf, 136.45 KBytes) |
| Attachment 2 | Competing interests (000348_Attachment2.pdf, application/pdf, 107.29 KBytes) |




