Intraoperative objective measurements in synaptopathy/neuropathy: Extra-/intracochlear ECochG monitoring and E-CAP/-ABR diagnostics in cochlear implant recipients
Bagas Marwan 1Silke Helbig 1
Martin Leinung 1
Anette W. Weiss 1
Timo Stöver 1
Tobias Weissgerber 1
Uwe Baumann 1
1 Goethe University Frankfurt, University Hospital, ENT-Department, Frankfurt am Main, Germany
Abstract
The provision of a cochlear implant (CI) effectively helps patients with sensorineural hearing loss (SNHL). Auditory neuropathy spectrum disorder (ANSD) is a rare cause of SNHL and may affect CI outcome. A total of three patients, comprising one adult and two pediatric cases, exhibited indications suggestive of bilateral functional deafness caused by auditory neuropathy spectrum disorder (ANSD). Consequently, each of these patients was provided with a cochlear implant (CI) in both ears. Prior to CI surgery, auditory brainstem responses (ABR) and transtympanic electrocochleography (ECochG) were performed to confirm the diagnosis of ANSD. Intracochlear ECochG with either pure tone burst or broadband chirp was performed on each patient during electrode insertion via the implant electrode array. E-ABR recordings and measurement of electrical compound action potentials (E-CAP) were performed sequentially. Preoperative ECochG and ABR showed the presence of cochlear microphonics (CM) but the absence of CAP. The hypothesis underlying the study is that in ANSD the CM is detectable and the CAP and the ABR waves J1 and J5 are absent, whereas in hearing supported or restored by a CI all of these responses are present. Intraoperative measurements facilitate a differential diagnosis of perisynaptic disorders (postsensory and pre-neural) and allow for an immediate determination at the conclusion of the surgical procedure that CI fitting for these patients is highly probable to be successful.
Keywords
auditory neuropathy, cochlear implant, intracochlear EcochG
1 Introduction
Auditory neuropathy spectrum disorder (ANSD) is a rare cause of sensorineural hearing loss (SNHL) in which the affected individual retains functioning outer hair cells in the cochlea. However, the inner hair cells are unable to respond to acoustic signals and transmit them to synaptic fibres or spiral ganglion neurons [1]. The presence of ANSD can be identified through the observation of abnormalities in audiological measurements where the otoacoustic emissions (OAE) remain intact. Furthermore, electrocochleography (ECochG) exhibits cochlear microphonics (CM), yet lacks the emergence of compound action potentials (CAP)/Jewett Wave I. Additionally, auditory brainstem responses (ABR) exhibit dysmorphism/flattening on Jewett Wave V. The treatment of ANSD can be challenging due to the disruption of sound coding in the auditory neural pathway. The use of amplification, such as that provided by hearing aids, to enhance sound stimuli is unlikely to yield significant benefits with regard to speech development and hearing perceptions [2]. Cochlear implantation represents a potential therapeutic intervention for patients with ANSD, as it converts sound into coded electrical signals, bypassing the hair cells and transmitting them directly to the spiral ganglion cells. A number of studies have documented encouraging results in patients with ANSD who have undergone cochlear implantation [2], [3], [4].
In addition, preserving the function and structure of the cochlea is crucial, especially to reduce the risks of mechanical damage [5]. Intracochlear ECochG recording may help to monitor inner ear damage by assessing acoustic evoked potentials from the hair cells of the cochlea and compound action potentials (CAP) from the auditory nerve fibers by using the CI electrode to record the cochlear response to acoustic stimuli.
The present study reports the collection of objective diagnostic and monitoring data, including ABR and ECochG, in patients with suspected bilateral ANSD. Moreover, the study illustrates the suitability of transtympanic and intracochlear ECochG for assessing the presence or absence of auditory neuropathy. It is of particular importance to note that the application of ECochG recordings during the insertion of the electrode array via the most apical electrode allows for the observation of CM characteristics, thus potentially preserving the integrity of cochlear structures.
2 Methods
2.1 Subjects
The study was conducted at the Department of Otorhinolaryngology of the Goethe University Frankfurt am Main, Germany. The study included three patients with a diagnosis of bilateral ANSD (Table 1 [Tab. 1]).
The newborn screening test for otoacoustic emissions was passed by both P2 and P3. P1 exhibited distortion product otoacoustic emissions in both ears in preoperative tests. After administering of general anesthesia for CI surgery, extracochlear ECochG tests were performed using the ECLIPSE system (Interacoustics A/S, Middelfart) with a disposable needle electrode inserted transtympanically into the promontory of the cochlea. The impedances of the needle electrode were checked prior to recording. EEG electrodes were placed on the right and left mastoid and forehead for the recordings. The results of the extracochlear ECochG tests, conducted with either an alternating pure tone burst at 1 kHz (P1, P3) or a click (P2), at sound levels between 80 and 100 dB HL, indicated the presence of CM. However, the compound action potentials (CAP/Jewett Wave I) were absent (see Figure 1 [Fig. 1]). Moreover, ABR responses to 0.25–4 kHz pure tone pulses were either dysmorphic or absent, even at 90 dB HL (data not shown).
Figure 1: Extracochlear ECochG (recorded with a transtympanic needle electrode) of P1–P3, either as a click (P1R, P2R, P2L) or as a pure tone burst at 1 kHz (P1L, P3R, P3L), with a sound level of 80–100 dB HL. Same pattern in all patients: CM exhibited high amplitude, while the CAP response was either absent or low. Color coding: CM (green), CAP (blue: left ear, red: right ear)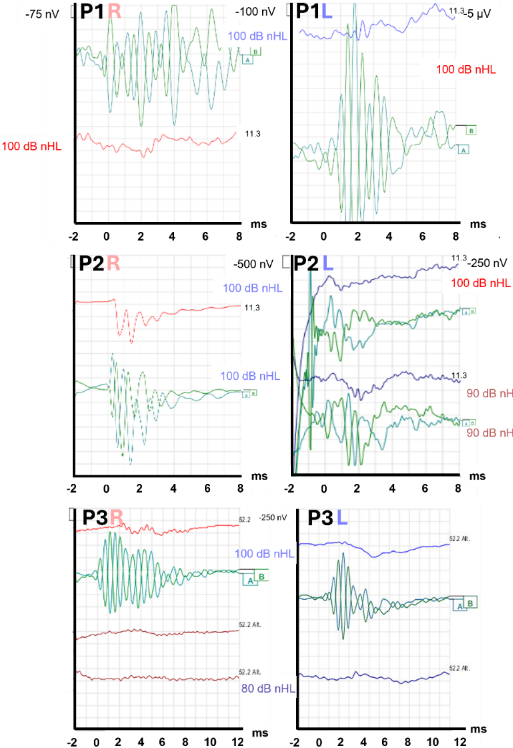
2.2 Intraoperative objective measurements
The CI electrode lengths were selected for each patient on an individual basis, based on the depth of the cochlea, using the Otoplan program (CASCINATION AG, Bern, Switzerland). P1 and P3 received Flex28 electrodes, while P2 received FlexSOFT electrodes (MED-EL, Innsbruck, Austria), which were inserted via the round window. During electrode insertion, intracochlear EcochG was performed using Maestro 904 v 4.1 (MED-EL, Innsbruck, Austria) R&D software and a Dataman system (Dataman Programmers Ltd., Dorset, UK) to generate acoustic stimulation (Figure 2 [Fig. 2]), as previously described [6], [7]. A broadband chirp (0.25–4 kHz, stimulus duration 12 ms, measurement window 12.8 ms) was performed on P1 and P3, while in P2 a 500 Hz pure tone burst with a stimulus duration of 8 ms and a recording window of 9.7 ms was applied. The stimulation was delivered via E-A-RLink insert earphones. Stimuli were employed to evaluate the electrophysiological response as a function of insertion depth along the cochlea using intracochlear ECochG. Initial ECochG recordings during insertion were performed with the most apical electrode (E1) and the back-telemetry measurement system of the implanted device [8]. Once complete insertion had been achieved, ECochG recordings were conducted on all electrodes (sweep recording).
Figure 2: Preparation for intracochlear EcochG measurements via CI. The telemetry coil is positioned on the implant, and the earphone is inserted on the side designated for recording. The trigger signal (MAX programming interface) is relayed to the Dataman 531 AWG, which is responsible for generating the acoustic stimuli. Subsequently, these signals are transmitted to the earphones. 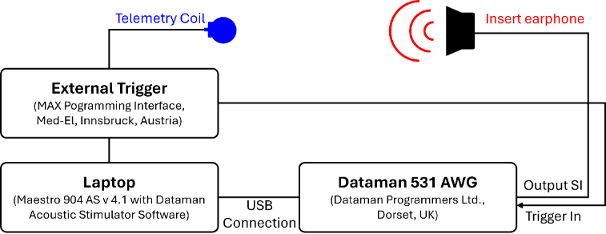
Upon completion of the intracochlear ECochG recording, a series of standard telemetry measurements were performed on the implant, as part of the post-procedure assessment. These included impedance measurement, electrically evoked stapedius reflex threshold (ESRT), and electrically evoked compound action potentials (E-CAPs) utilising AutoART (MED-EL, Innsbruck, Austria) [9].
Finally, electrical auditory brainstem responses (E-ABR) were recorded to investigate the integrity of the auditory nerve and brainstem. The trigger output of the MAX Programming Interface (MED-EL, Innsbruck, Austria) was connected to the trigger input of the ECLIPSE system. Once the appropriate impedances had been confirmed, the electrophysiological responses were recorded with EEG electrodes. Alternating biphasic electrical pulses were delivered to selected apical, medial, and basal electrodes with a duration of 40 µs each. The stimulus rate was set to 34 Hz, and 500 recordings were made for each measurement.
3 Results and discussion
In all patients, multiple frequency-specific CM (e.g., 250 Hz, 500 Hz, 1 kHz, 2 kHz, and 4 kHz) were identified during the insertion of the CI electrode (Figure 3A [Fig. 3], data P3 not shown). P3 is not shown in Figure 3 [Fig. 3] as results are similar. Moreover, CM were still present after the complete insertion of the CI electrodes (Figure 3C [Fig. 3]), indicating that the mechanics of the outer hair cells were still functioning effectively. Additionally, phase changes in CM amplitude (Figure 3C [Fig. 3], panel 2) and increased/dropped FFT amplitude at a given frequency, which were dependent on the depth of the electrode, were also observed. Further investigation is necessary to determine the relationship between these changes and to identify any dependencies on electrode insertion progress.
Figure 3: Intracochlear electrocochleographic (ECochG) recording during the insertion of the electrode P1 (1) and P2 (2) (screenshot, Maestro 904 v 4.1 (MED-EL, Innsbruck, Austria)). Upper: Broadband chirp stimulus, 97 dB HL. Lower: 500 Hz pure tone burst, 115 dB HL. A. CM amplitude during insertion, recording electrode 1. The frequency-specific CM amplitude was estimated by means of a Fast Fourier Transformation (FFT) of the CM response (C) from the beginning to the end of the insertion. Color coding: red 250 Hz, yellow 500 Hz, light green 1 kHz, dark green 2 kHz, cyan 4 kHz. Panel A: Labels with numbers indicate depth of electrode insertion. E12 marks the point of complete insertion. Panel B: Response latency during insertion. Panel C: CM responses (recording electrode 1) during electrode insertion, commencing from the top (four electrodes inserted in P1; six electrodes inserted in P2) and progressing to the bottom. CM responses are typically sustained even after the complete electrode has been inserted (data for P3 not shown as results are similar).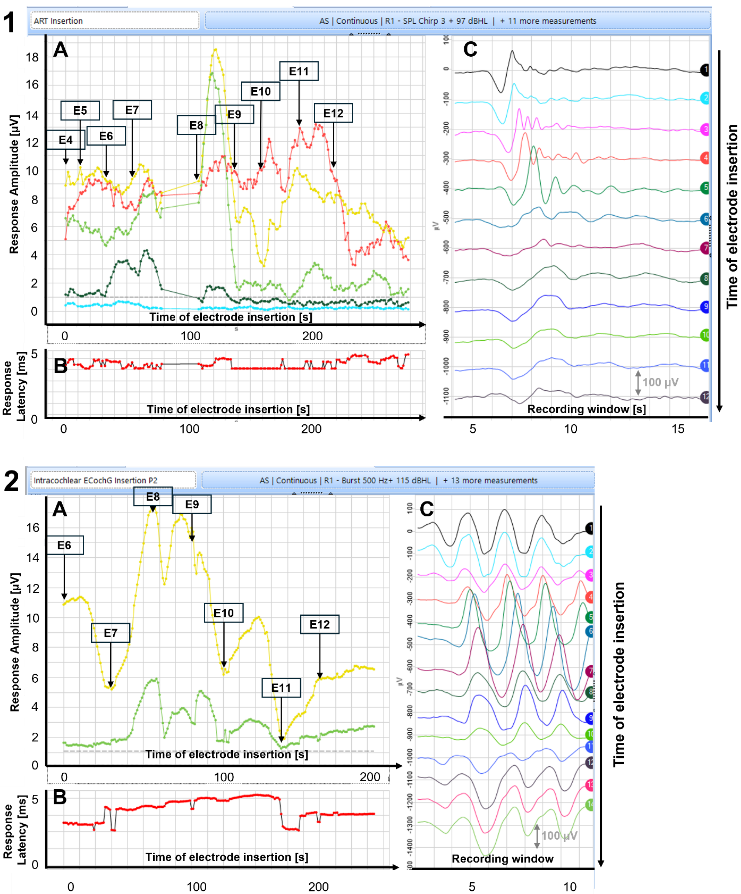
Figure 4 [Fig. 4] illustrates exemplary the E-ABR response of P2 (P1/P3 not presented here) with the recording electrodes positioned at E2 (apical), E5 (medial) and E10 (basal). In P2, electrical pulses between 600 and 1,200 cu with an interval of 200 cu were applied for each electrode. The results demonstrate that the amplitude of the Jewett wave V exhibited an increase with the application of increasing electrical stimulation. In addition, the neural thresholds for P2 can be approximated to be between 600 and 800 cu (Figure 4 [Fig. 4]). This suggests that the electrical stimulation successfully stimulates auditory structures in the brainstem, thus enabling subsequent processing and development of speech perception abilities.
Figure 4: E-ABR responses P2. A: apical (E2). B: medial (E5). C: basal (E10). Stimulation electrode: neighbouring electrodes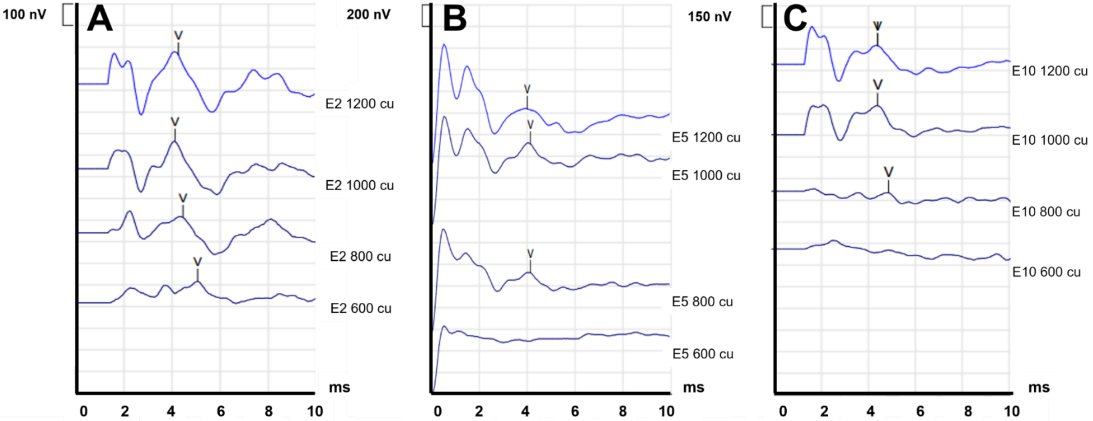
The efficacy of cochlear implantation (CI) for the rehabilitation of patients diagnosed with ANSD, when the exact location of lesions is unclear, is a subject of ongoing debate among experts in the field [10]. The efficacy of CI is reduced when the lesion is located at the level of the nerve itself, such as in cases of cochlear nerve deficiency (CND)/postsynaptic lesions, as opposed to within the cochlea or at the synapse between nerves/presynaptic lesions [3], [11], [12]. The intraoperative measurements enable a differential diagnosis of the perisynaptic (postsensory but preneural) disorder and lead to the conclusion directly at the end of the surgical procedure that the CI fitting of the patient affected by ANSD is very likely to be successful.
Figure 5 [Fig. 5] depicts the intracochlear ECochG results (P1) in sweep electrode mode (stimulus: broadband chirp). Each electrode (E1-E12) is employed for CM-recording. Interestingly, the results at four months post-surgery demonstrate an enhancement in the CM response to 250 Hz (panel 2A, red line), when recording at basal electrodes E10-E12. In contrast, the responses to 1 kHz (green line) showed a notable reduction at E10.
Figure 5: Upper: Intracochlear ECochG sweep recording (E1 to E12, P1, intraoperatively). Lower: four months post surgery. Broadband chirps were used as acoustic stimuli. Panel A: CM FFT-Amplitude E1 (left) to E12 (right). Color coding: red 250 Hz, yellow 500 Hz, light green 1 kHz, dark green 2 kHz, cyan 4 kHz. B: response latency. C: CM recordings of E1–E12 from top to bottom (sweep electrode)
4 Conclusion
The cases presented here demonstrate that CI implantation represents a viable treatment option for rehabilitating patients with presynaptic disorders, such as synaptopathy and dysfunction of the inner hair cells. ANSD was characterized by normal CM with absent CAP/Jewett Wave I in the ECochG test and missing Jewett wave V in the ABR test. While ECochG can be employed to substantiate the diagnosis of ANSD, it is not a sufficient method for determining the precise location of lesions. The E-CAP test after CI provision is a straightforward method for evaluating the integrity of the auditory nerve (Jewett Wave I)/spiral ganglion. In addition, the E-ABR is a valuable diagnostic tool that allows examination of the auditory pathway, from the cochlea to the brainstem, with the objective of ensuring the electrical stimuli reach the brainstem, following the insertion of the CI electrode. Furthermore, intracochlear ECochG monitoring offers valuable insights for surgeons and audiologists in the assessment of cochlear health, particularly with regard to the preservation of residual hearing. Nevertheless, the results remain open to interpretation and are not yet suitable for reliable hearing monitoring during surgery. This report presents a preliminary account of a few initial clinical observations, which, by their nature, cannot fulfill the requirement of systematic completeness. Further studies are required to investigate the relationship between changes in the ECochG response and insertion trauma leading to hearing loss.
Notes
Conference presentation
This contribution was presented at the 26th Annual Conference of the German Society of Audiology and published as an abstract [13].
Competing interests
The authors declare that they have no competing interests.
References
[1] Moser T, Starr A. Auditory neuropathy--neural and synaptic mechanisms. Nat Rev Neurol. 2016 Mar;12(3):135-49. DOI: 10.1038/nrneurol.2016.10[2] Trautwein PG, Sininger YS, Nelson R. Cochlear implantation of auditory neuropathy. J Am Acad Audiol. 2000 Jun;11(6):309-15.
[3] Sarankumar T, Arumugam SV, Goyal S, Chauhan N, Kumari A, Kameswaran M. Outcomes of Cochlear Implantation in Auditory Neuropathy Spectrum Disorder and the Role of Cortical Auditory Evoked Potentials in Benefit Evaluation. Turk Arch Otorhinolaryngol. 2018 Mar;56(1):15-20. DOI: 10.5152/tao.2017.2537
[4] Buss E, Labadie RF, Brown CJ, Gross AJ, Grose JH, Pillsbury HC. Outcome of cochlear implantation in pediatric auditory neuropathy. Otol Neurotol. 2002 May;23(3):328-32. DOI: 10.1097/00129492-200205000-00017
[5] Skarżyński PH, Lorens A, Walkowiak A, Polak M, Skarżyński H. Multi-Frequency Intraoperative Monitoring of Hearing Preservation during Cochlear Implantation. Life (Basel). 2022 Apr;12(5):636. DOI: 10.3390/life12050636
[6] Schuerch K, Waser M, Mantokoudis G, Anschuetz L, Wimmer W, Caversaccio M, Weder S. Performing Intracochlear Electrocochleography During Cochlear Implantation. J Vis Exp. 2022 Mar;(181):e63153. DOI: 10.3791/63153
[7] Skarżyński PH, Lorens A, Walkowiak A, Polak M, Skarżyński H. Multi-Frequency Intraoperative Monitoring of Hearing Preservation during Cochlear Implantation. Life (Basel). 2022 Apr;12(5):636. DOI: 10.3390/life12050636
[8] Baumann U, Stöver T, Weißgerber T. Device profile of the MED-EL cochlear implant system for hearing loss: overview of its safety and efficacy. Expert Rev Med Devices. 2020 Jul;17(7):599-614. DOI: 10.1080/17434440.2020.1781614
[9] Schrank L, Nachtigäller P, Müller J, Hempel JM, Canis M, Spiegel JL, Rader T. ART and AutoART ECAP measurements and cochlear nerve anatomy as predictors in adult cochlear implant recipients. Eur Arch Otorhinolaryngol. 2024 Jul;281(7):3461-73. DOI: 10.1007/s00405-023-08444-5
[10] Shearer AE, Hansen MR. Auditory synaptopathy, auditory neuropathy, and cochlear implantation. Laryngoscope Investig Otolaryngol. 2019 Aug;4(4):429-40. DOI: 10.1002/lio2.288
[11] Bayri Ulukan M, Ciprut A. Intracochlear electrocochleography findings in cochlear implant recipients with auditory neuropathy spectrum disorder. Int J Pediatr Otorhinolaryngol. 2023 Jul;170:111596. DOI: 10.1016/j.ijporl.2023.111596
[12] Riggs WJ, Roche JP, Giardina CK, Harris MS, Bastian ZJ, Fontenot TE, Buchman CA, Brown KD, Adunka OF, Fitzpatrick DC. Intraoperative Electrocochleographic Characteristics of Auditory Neuropathy Spectrum Disorder in Cochlear Implant Subjects. Front Neurosci. 2017;11:416. DOI: 10.3389/fnins.2017.00416
[13] Marwan B. Intracochlear ECochG in synaptopathy/neuropathy: What do cochlear microphone potentials tell us? In: Deutsche Gesellschaft für Audiologie e.V., editor. 26. Jahrestagung der Deutschen Gesellschaft für Audiologie. Aalen, 06.-08.03.2024. Düsseldorf: German Medical Science GMS Publishing House; 2024. Doc089. DOI: 10.3205/24dga089