Rehabilitation of the lower extremities, standing and walking function in people with spinal cord injury or disease: Guideline of the German-Speaking Medical Society for Spinal Cord Injury
Sophie Irrgang 1Sandra Himmelhaus 1
Kirstin Allek 2
Claudio Bartholet 3
Ines Bersch-Porada 4
Armin Curt 3
Burkhart Huber 5
Daniel Kuhn 6
Karen Kynast 7
Norbert Weidner 7
Anke Scheel-Sailer 1,8
1 Swiss Paraplegic Research, Nottwil, Switzerland
2 Spinal Cord Injury Center, Zentralklinik Bad Berka, Germany
3 Spinal Cord Injury Center, University Hospital Balgrist, Zurich, Switzerland
4 Swiss Paraplegic Center, Nottwil, Switzerland
5 Spinal Cord Injury Rehabilitation Center, Bad Häring, Austria
6 Clinic for Physical and Rehabilitative Medicine, Berufsgenossenschaftliche Kliniken Bergmannstrost, Halle, Germany
7 Spinal Cord Injury Center, Heidelberg University Hospital, Heidelberg, Germany
8 Centre for Rehabilitation and Sport Medicine, Insel Group, University Bern, Switzerland
Abstract
Introduction: According of the level and severity of the spinal cord injury or disease (SCI/D), and the impairment of motor, sensory, and autonomic functions, individuals with SCI/D recover some standing and walking capabilities. To increase quality of rehabilitation and use newest evidence, the clinical practice guideline (CPG) “S2e-Guideline Rehabilitation of lower extremities, standing and walking function in people with SCI/D” of the German speaking Medical Association for Paraplegiology (DMGP) was updated.
Methods: Following a multi-tiered approach systematic searches were conducted to identify appropriate literature. For this purpose, the Databases PubMed, EMBASE, Cochrane Library and PEDro were searched. Recommendations on assessments were grouped to the categories “activity and participation” or “body functions/body structures”. Recommendations on interventions were labeled with outcomes standing, walking, strength, range of motion, pain and muscle tonus.
Results: In total, 9,871 studies were identified during the search. Of these, four systematic reviews and eleven primary studies were utilized in composing the recommendations. A total of 25 recommendations were made, with 20 derived from the literature and 5 based on expert consensus. In total 14 functional assessments and 11 rehabilitation interventions became compiled. The assembled recommendations regarding assessments could be well built on published literature, while overall there is a paucity of literature proofing the evidence of specific interventions used in clinical practice. Therefore, the expertise of the international expert group and input from patient representatives were pivotal.
Conclusion: The method of an evidence-based guideline was sufficient for the recommendation of functional assessments but showed the need scientific clarification in the field of clinically established interventions.
Keywords
spinal cord injury, rehabilitation, lower extremities, standing, walking
1 Introduction
A spinal cord injury or disease (SCI/D) can result in motor, sensory, and autonomic dysfunctions, significantly impacting the physical, psychological, and social well-being of affected individuals. The extent of these dysfunctions varies widely, from minimal impairment to complete loss of function below the lesion level. Therefore, depending on the level and severity of the spinal cord lesion, individuals with SCI/D may regain the ability to stand and walk [1], [2]. As a rare health condition, the population-adjusted incidence rate of SCI/D in Germany is 15.727 per million per year [3] and due to the complexity of impairment patterns usually rehabilitation of individuals with a SCI/D is best provided in specialized SCI centers. An evidence-based development of guidelines might help to summarize existing evidence, contextualize the evidence within the cultural framework of the national context and inform clinical practice across the continuum of care with the best recommendation for coordinating and choosing rehabilitation interventions.
The guideline titled “Rehabilitation of the Lower Extremity, Standing, and Walking Function after Spinal Cord Injury” is a first update of the German speaking Society of Paraplegia (DMGP) in the framework of the Association of the Scientific Medical Societies in Germany (AWMF) and provides recommendations for the rehabilitation of patients with both complete and incomplete SCI/D across acute, subacute, and chronic phases.
The recommendations address the patient’s functional capacity according to the “International Classification of Functioning, Disability and Health” (ICF) [4] and not on the level of lesion or the extent of motor impairment according to the “International Standards for Neurological Classification of Spinal Cord Injury” (ISNCSCI) [5]. Given that rehabilitation is based on the ICF concept, this guideline takes a particular interest in the assessment of functionality and incorporates it into the formulation of recommendations for rehabilitation interventions [6]. This allows for the planning of rehabilitation across the continuum of care.
Primarily, the guideline offers recommendations for adults, though certain recommendations can be adapted for children and adolescents as needed. Although children have different requirements, lesion characteristics and dynamic of recovery the chance to develop a guideline is even less, due to the gap of scientific publications related to the rare health condition [7]. Recognizing that functional changes may occur due to neurological recovery or based on adaptation/compensation [8], the rehabilitation process, including the selection of appropriate assessments and interventions, remains dynamic [9]. The quality of rehabilitation is enhanced through a tailored selection of interventions based on the patient’s needs, common goal formulations [10], [11], and considerations of restoration, neuroplasticity, neuromodulation, and neuroregeneration [12], [13]. Additionally, interventions may be chosen to address or prevent complications in secondary prevention [14].
Although a guideline will provide recommendations for assessments and interventions, the adherence to specific recommendations will depend on the responsible physicians and therapists, taking into account the patient’s condition, existing circumstances, and available resources [15], [16]. Because the rehabilitation of the lower extremity follows comparable treatment principles the guidelines are applicable across various care settings, including outpatient care, day-care, inpatient care, rehabilitation, specialized care, and lifelong aftercare for individuals with SCI/D [17]. The recommendations set forth in the guideline are intended for implementation by medical specialists, physiotherapists, and occupational therapists.
The aim of this guideline is to recommend assessments to measure standing or walking function and interventions to maintain or optimize standing or walking function in individuals with spinal cord injury or disease across the continuum of care.
2 Methods
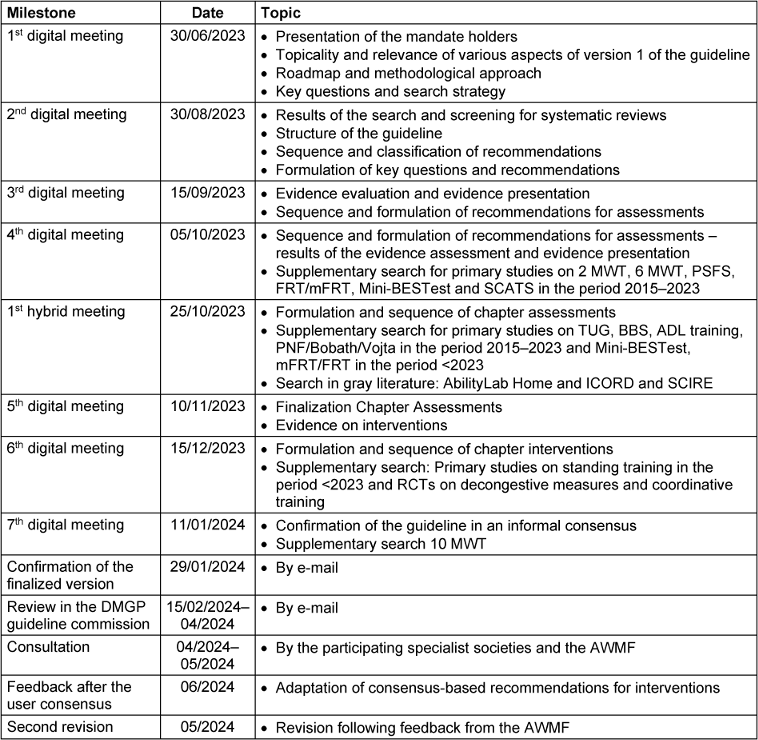
The methodology applied for the guideline preparation was aligned in the framework of the “AWMF”. The literature search was conducted systematically, similar to the approach used in systematic reviews [15]. The initial methodological analysis of the topic was carried out by two research assistants with therapeutic expertise. This systematic literature search ensured that the guideline was classified as an evidence-based guideline (Se2 guideline). Subsequent to the literature selection, the content was reviewed and refined by a multidisciplinary expert group. The precise timetable is outlined in Table 1 [Tab. 1].
The expert group comprised nine professionals, including physiotherapists, occupational therapists, neurologists, orthopedic surgeons and rehabilitation physicians, delegated from the DMGP.
2.1 Literature search
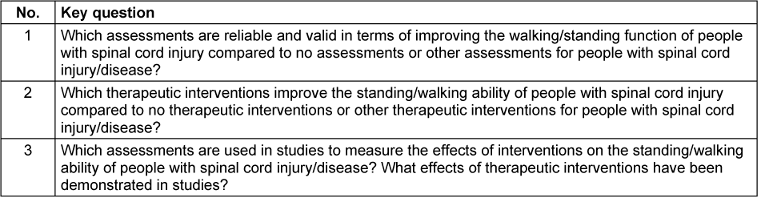
On March 29, 2023, the databases PubMed, EMBASE, Cochrane Library, and PEDro were searched for systematic reviews published since the completion of Version 1 (2015–2023). The basis for this search were the a priori defined key and research questions (Table 2 [Tab. 2]).
Search terms included text words and subject headings (MeSH). The following MeSH terms were used: “spinal cord injur*”, “paraplegia”, “assistive devices”, “therapeutic interventions”, “assessment”, “muscle strength”, and “walk*”. The search was complemented by screening references of the literature found and the screening of grey literature.
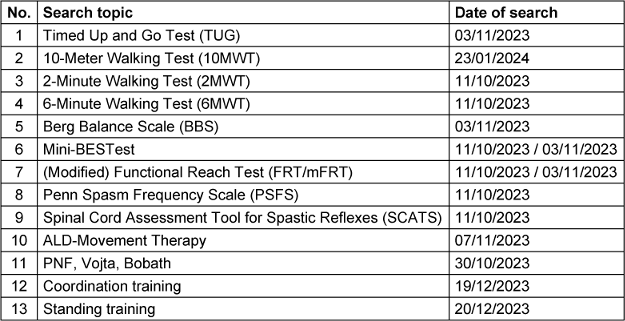
Due to a lack of literature found during the search for systematic reviews, 16 additional searches for primary literature were conducted in conjunction with the initial search for systematic reviews (Table 3 [Tab. 3]).
These searches targeted studies on specific topics related to interventions or assessments. The basic text words and MeSH terms remained consistent across these searches, with additional terms incorporated to refine and specify the topics of interest.
The comprehensive search strategy for systematic reviews and the searches for primary studies can be found in the Appendix 1 in Attachment 1 [Att. 1].
2.2 Eligibility criteria
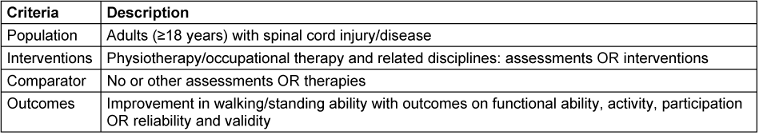
The eligibility criteria for this literature search were defined according to the PICO-framework [18] and are presented in Table 4 [Tab. 4].
For all searches, the inclusion criteria were defined as: adults (≥18 years) diagnosed with SCI/D, human studies, assessments to quantify the standing and walking function, functioning assessments, as well as validity, reliability and objectivity, therapeutic interventions to maintain or improve standing and walking function, outcomes on body functioning (strength, mobility, endurance, balance, fine and gross motors skills). Reasons for exclusion were children <18 years), other diseases, animal studies, surgical or pharmacological interventions, biochemical outcomes, other study designs. During the additional searches, these inclusion and exclusion criteria were partially specified and adapted to the specific intervention or assessment.
2.3 Selection process
The selection of retrieved studies was based on predefined inclusion criteria. The tool Rayyan was employed to streamline the selection process [19]. Two trained and blinded researchers (SI, SH) independently screened the studies for matching titles, abstracts, and full texts. In cases of ambiguity, an expert (ASS) was consulted to make the final decision on study inclusion or exclusion.
2.4 Analysis of study quality
The quality of the retrieved studies was assessed using the checklists required by the AWMF [15]. The quality of the available literature was evaluated using different tools.
To assess the quality of systematic reviews, the MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR 2) tool was used [20].
To evaluate case-control studies and cross-sectional studies, the Newcastle Ottawa Quality Assessment Scale for Case Control Studies (NOS) was used [21].
Randomized controlled trials (RCTs) were assayed using the Cochrane risk-of-bias tool for randomized controlled trials (RoB 2) tool [22].
Reliability studies were reviewed using the COSMIN Risk of Bias Tool [23].
To finally determine the level of evidence for each included literature, the Oxford Levels of Evidence 2011 were used [24]. Classification is based on a scale of 1 to 5, with the best possible evidence level being represented by a 1.
These evaluations were conducted independently by two blinded reviewers (SI, SH) and subsequently compared. Any discrepancies were resolved through discussion with an expert (ASS).
2.5 Data collection and synthesis of results
An evidence table was created for each PICO question, detailing assessments and interventions (Appendix 2 in Attachment 1 [Att. 1]). These tables contain information on the references of the included studies, the PICO elements, the main results, and the critical evaluation of the evidence. The assessments were classified into two categories to facilitate a more precise subdivision. The initial category encompassed assessments pertaining to “activity and participation,” whereas the subsequent category encompassed assessments pertaining to “body functions/body structures.” This categorization is based on the classification system outlined in the ICF model.
To address the third research question, outcomes recorded in the studies were systematically categorized and labeled according to the underlying research question. Outcome labels were assigned to the corresponding recommendation boxes for the interventions, indicating which effects have been explicitly scientifically investigated and validated.
2.6 Formulation of recommendations
Based on the comprehensive literature review, a collaborative exchange was conducted among all participating experts to develop the recommendations. The precise wording and the levels of recommendation were assigned accordingly. The assignment of recommendation levels considered methodologically prepared evidence, clinical experience, relevance and feasibility, consistency of study results, and their applicability to the target patient group and their preferences. Recommendations were categorized as strong (A), standard (B), or open (C). Throughout this process, the expert group systematically evaluated the benefits, side effects, and risks associated with each recommendation.
2.7 Participation of patients
As no patients were involved during the development of the guideline, a focus group discussion was conducted with patient representatives following the guideline’s completion. Patient representatives from Germany and Switzerland participated. This patient group provided various suggestions for modifications to the recommendations, primarily regarding the wording and the levels of recommendation. The expert group reviewed these proposed changes and subsequently incorporated them into the guideline.
3 Results
3.1 Study selection
The comprehensive search for systematic reviews yielded 4,374 articles. Following the screening of titles and abstracts, 285 articles were selected for full-text review, resulting in the inclusion of 49 systematic reviews in the guideline. Additional searches for primary literature produced 5,771 results, of which 59 articles underwent full-text screening. Ultimately, 22 primary studies were included in the guideline.
After expert group revisions, four systematic reviews and eleven primary studies were utilized to address the key questions and formulate the recommendations. An overview of the selection process is presented in Figure 1 [Fig. 1], and detailed flow charts for the individual searches are available in the supplementary material (Appendix 1 in Attachment 1 [Att. 1]). A total of 25 recommendations were made, with 20 based on the literature and five derived from expert consensus.
Figure 1: PRISMA flow diagram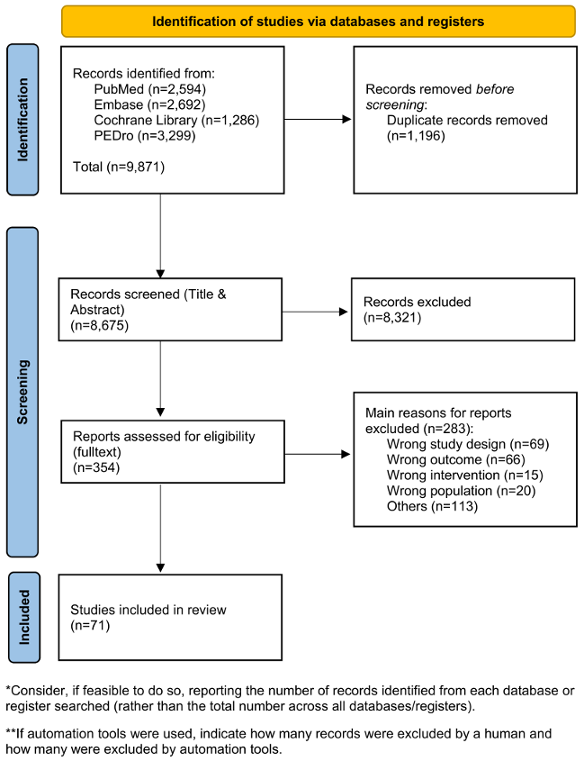
Adapted from Page et al. [67], licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/)
3.2 Assessments
Explanation of the recommendations
In this guideline, only assessments that meet psychometric quality requirements (validity, reliability) and align with the guideline’s objectives (walking and standing function) were included. The recommendations from the guideline on outcome assessment in primary treatment [25] served as the foundation for content selection. Additionally, assessments used in systematic reviews of interventions to improve walking and standing function were documented. This guideline does not aim to evaluate all assessments used in specific studies or routine clinical practice.
In addition to the listed examinations, assessments, and measurement methods, general principles were described to underline the quality framework: a clinical examination based on medical history, inspection, and palpation is essential for individualized and specific treatment. This includes evaluating reflex status, joint examination (including stability), muscle lengths, depth sensitivity, and pain assessment [26], [27]. Joint status encompasses the range of motion (ROM) and should be performed using the neutral zero method (NNM). The manual muscle function test (MMT) should be conducted regularly, especially if there is clinical indication of muscle strength changes [28]. The MMT and joint mobility assessments are tailored to the paralysis pattern and the patient’s specific situation [29]. Muscle lengths are measured and described using established methods [30]. Leg length should be measured if clinically indicated (e.g., leg length discrepancy) for patients with SCI/D. Pain assessment should follow the recommendations of the guideline on pain in paraplegia [31]. Depth sensitivity is assessed using the tuning fork test (vibration) [26].
The examinations, assessments, and measurement methods are recommended for all phases: acute (up to 2 weeks after SCI/D onset); subacute (3 weeks to 6 months after SCI/D onset or initial treatment/rehabilitation); and chronic (longer than 6 months after SCI/D onset or post-discharge from initial inpatient treatment) [32]. Assessments should be consistently conducted at admission and discharge or at the start and end of outpatient, day-care, or inpatient rehabilitation, during annual reviews, or when there is clinically observed deterioration in functional capacity. Unless otherwise stated, all assessments are applicable to patients with both complete and incomplete SCI/D.
In special cases, additional assessments may be utilized [33]. Consequently, the Spinal Cord Injury Functional Ambulation Inventory (SCI-FAI), previously given a “may” recommendation in the first version of the guideline, is no longer included. Following the overarching rehabilitation objectives, assessments for activities and participation are prioritized, followed by assessments for structure and function.
The recommendations for the assessments are presented in Table 5 [Tab. 5] and Table 6 [Tab. 6]. They are organized into two categories: recommendations pertaining to the topic of “activity and participation” and recommendations pertaining to the topic of “body functions/body structures.”
Table 5: Recommendations for assessments: activity and participation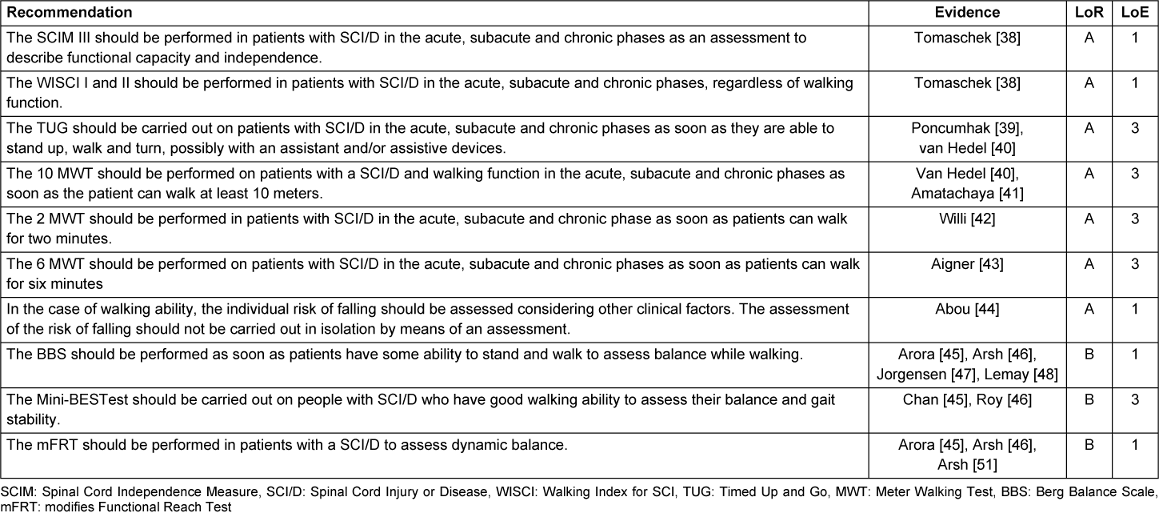
Table 6: Recommendations for assessments: body functions/body structures
3.3 Interventions
Explanation of the recommendations
When describing interventions the following five topics evolved as relevant for the individualized adaptation of interventions:
Selection of interventions
The selection of interventions for individuals with SCI/D is influenced by several key aspects:
- The person with SCI/D with their biological, psychological and social circumstances
- The expected complications in the context of SCI/D
- The jointly defined goals in terms of improving functional capacity
- The organizational and structural conditions in the cross-sectional center
Individual with SCI/D: bio-psycho-social considerations
Interventions are tailored to the sensorimotor, neurological, and medical capabilities of the individual, addressing their functional mobility limitations, cognitive impairments, and complications such as pain and spasticity. Additionally, patient-specific factors such as exhaustion and fatigue are integrated into the individualized therapy design.
Anticipated complications in the context of SCI/D
The selection of treatments includes interventions aimed at preventing anticipated complications such as contractures, pain, or fractures.
Jointly defined goals for improving functional capacity
Goals should be collaboratively established with the patient, adhering to the SMART criteria (Specific, Measurable, Achievable, Relevant, Time-bound) [10]. The effectiveness of the intervention should be reassessed after a defined period using appropriate assessments [34]. Corresponding assessments should be conducted at the beginning and end of the therapy phase to evaluate the formulated goals or intermediate goals. Additionally, the guideline on outcome assessment in initial treatment after newly acquired spinal cord injury [25] should be referenced.
From a clinical perspective, interventions can directly or indirectly influence all of these aspects. In the table of recommendations, numbers refer to the specific aspects measured in scientific studies. Furthermore, if necessary, compensation mechanisms and the use of assistive devices are trained and adapted as needed.
Rehabilitation management and organizational aspects
Interventions should be selected by therapists experienced in treating SCI/D in routine clinical practice. Based on regional/focal and/or ADL-related movement therapy, which can be hands-on or hands-off with/without the use of aids, other forms of therapy are supplemented individually or in combination for limited periods, depending on individual goals, expected neurological improvements, and predominant health issues (e.g., spasticity, pain).
In the absence of evidence for individual components within the overall intervention of initial treatment, the institution and therapy team have the responsibility and flexibility to design a goal-oriented and individualized treatment plan.
Even when evidence suggests that increased intensity of active therapies, especially during the acute and subacute phases, associated with better recovery outcomes, lower limb rehabilitation must be integrated into the overall rehabilitation plan, considering other goals and therapeutic interventions, which may require compromises regarding ideal intensity.
Due to the unique situations and special needs of individuals with SCI/D, therapies should be generally conducted as individual sessions. Some interventions may be conducted in a group setting when appropriate.
This scientific approach ensures that interventions are optimally designed to address the complex needs of individuals with SCI/D, enhancing their functional capacity and overall quality of life.
All recommendations for the interventions are displayed in Table 7 [Tab. 7].
Table 7: Recommendations for interventions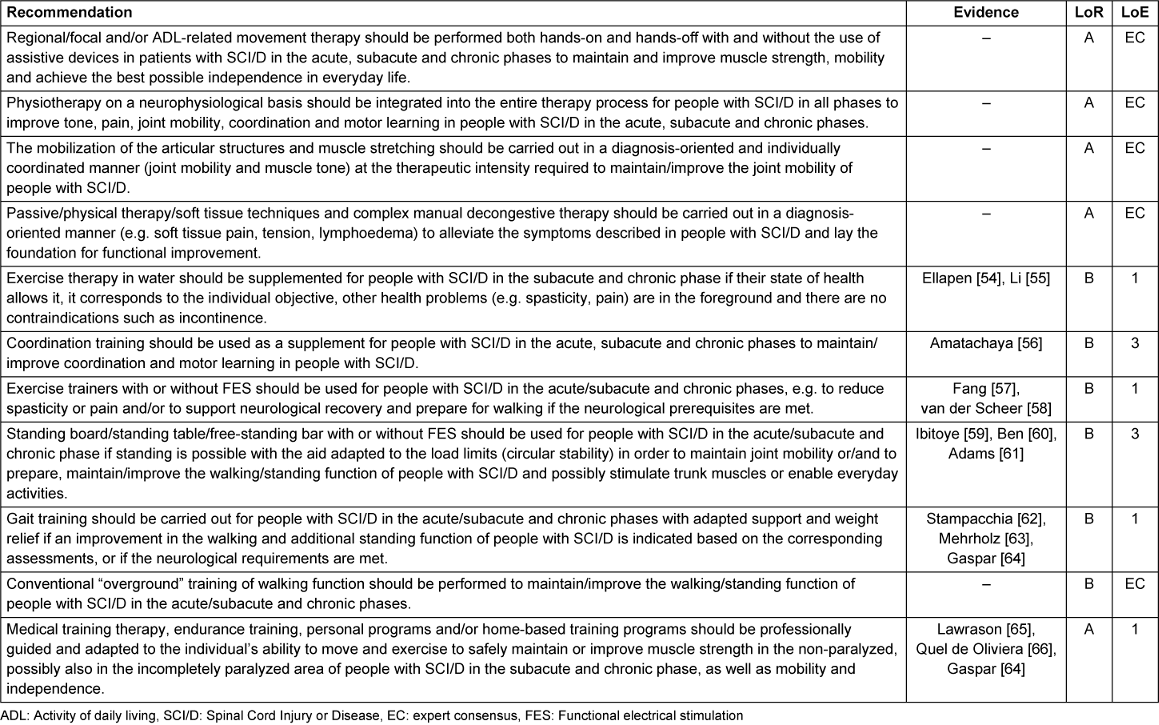
3.4 Outcomes
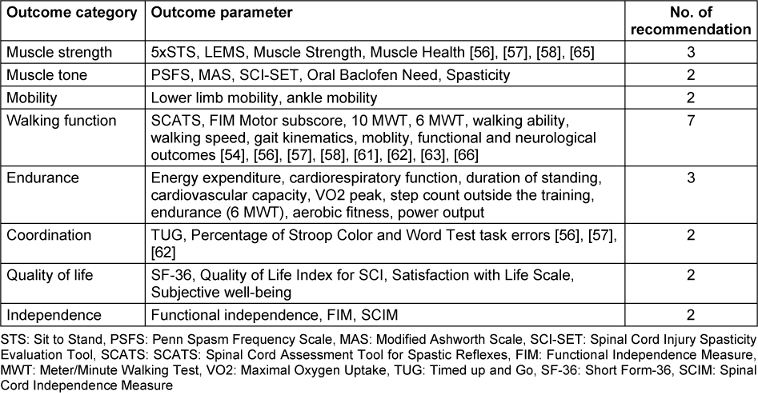
To address the third key question, all outcomes were systematically categorized and assigned specific labels. The expert group then applied these eight labels to the various identified outcome parameters and matched them with the appropriate recommendations. This process elucidates the quantity and nature of the recommendations that have been subject to scientific investigation and delineates their respective contexts. The majority of recommendations pertain to the outcome category of endurance, while the remaining categories each have two to three corresponding recommendations. Detailed information on all categories and their parameters is presented in Table 8 [Tab. 8].
Furthermore, these categories can be utilized to establish objectives that the patient can attain throughout the rehabilitation process. The aforementioned categories are not self-contained; thus, they can be utilized as standalone entities or in conjunction with one another throughout the course of therapy.
4 Discussion
Although sufficient literature has been identified regarding the assessment of functioning outlined in the recommendations, its quality varies widely, and suffers from significant limitations. Many studies feature small sample sizes, and comparability between studies is lacking.
Regarding rehabilitation interventions, literature was not available for all those utilized in clinical settings. As a result, some recommendations are based on expert consensus. However, a balanced combination of literature-based recommendations and those founded on the expert group’s practical experience and expertise was achieved.
The literature included in this guideline lacks specific treatment parameters such as number of repetitions or intensities, making it impossible to provide precise information in the recommendations. The available technical expertise was essential for developing recommendations applicable in everyday practice. The inclusion of patient representatives and the implementation of focus group discussions elevated the recommendation level from B to A for several recommendations for interventions. This highlights the critical importance of incorporating the patient perspective alongside professional expertise and literature-based knowledge.
The descriptions of the functions of walking and standing ability facilitated the presentation and description of assessments and interventions in a structured manner. This structure allows for the interconnection of discrete recommendations, thereby facilitating the formulation of a systematic sequence of assessments and interventions that are pertinent to routine clinical practice. This offers a significant advantage over other guidelines [35], [36], which merely list recommendations for walking and standing function without establishing a connection between them. As previously stated, the guideline can thus increasingly reflect everyday clinical practice, in which assessments and interventions are conducted with similar frequency and the interventions are based on the assessments performed. It is therefore important to view these recommendations as a unified set rather than as individual recommendations.
The following topics have emerged as important research questions for the rehabilitation of standing and walking function in people with SCI/D:
What interventions are used in different cross-sectional centers, e.g. in the acute and subacute phases after SCI/D?
The grid created to categorize assessments and interventions according to the ICF classification can serve as a basis for further observational studies. The aim could be to compare interventions in specific subgroups of people with SCI/D, considering the expected outcome of rehabilitation in different cross-sectional centers, to better understand rehabilitation of standing and walking function, and to develop targeted interventions to improve rehabilitation.
How effective are the consensus-based interventions recommended in this guideline?
All the consensus-based recommendations in this guideline could be a focus for research projects. The overall aim is to be able to base these recommendations on a strong evidence base in the future. This approach will enable continuous improvement in the care of people with SCI/D about their standing and walking function. By integrating evidence-based knowledge, future recommendations can be more precise and effective in improving the quality of life and functional ability of people with SCI/D.
What are the milestones in rehabilitation after the onset of SCI/D? Could they be used to guide the planning of rehabilitation?
Version 1 of this guideline presented a treatment pathway with milestones that had emerged from everyday clinical practice. As there is no evidence to support these milestones, this milestone plan has been dropped. Regarding the comparability of the overall intervention of initial treatment after SCI/D, it may be useful to investigate these milestones scientifically, possibly then subgroup-specific, to structure the treatment pathway and define goals.
5 Conclusion
This review underscores the significance of interdisciplinary cooperation in the development of clinical guidelines. The review revealed the existence of literature and studies pertinent to the rehabilitation of SCI/D though the quality of these sources is generally limited. Additionally, the guideline highlights a notable gap in high-quality studies addressing routine interventions, necessitating the inclusion of consensus-based recommendations despite the preference for evidence-based guidelines. To increase the quality of recommendation a S3 guideline including a structured consensus process might be better for future updates of the guideline.
Notes
Guideline
A full version and a comprehensive report in German language was published in the guideline portal of the AWMF [37].
Shared first authorship
Sophie Irrgang and Sandra Himmelhaus share first authorship.
Author contributions
The entire team contributed to the development of the underlying guideline. All authors collaboratively developed and approved the key and research questions. SI and SH were responsible for designing search strategies, screening the identified literature, assessing study quality, and collecting data. In cases of ambiguity, ASS made the final decision on study inclusion or exclusion. The decision to conduct additional searches for primary literature was made collectively during expert group discussions. SI prepared the initial manuscript draft, which SH and ASS reviewed and provided feedback on. All other authors reviewed and approved the final version of the manuscript.
Funding
Swiss Paraplegic Research, Nottwil, Switzerland, supported the development of the guideline by funding the two research assistants and providing materials and access to databases.
Competing interests
The authors declare that they have no competing interests.
References
[1] Fehlings MG, Tetreault LA, Wilson JR, Kwon BK, Burns AS, Martin AR, Hawryluk G, Harrop JS. A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: Introduction, Rationale, and Scope. Global Spine J. 2017 Sep;7(3 Suppl):84S-94S. DOI: 10.1177/2192568217703387[2] World Health Organisation; The International Spinal Cord Society, editors. International Perspectives on Spinal Cord Injury. Geneva: WHO, ISCOS; 2013.
[3] Rau Y, Schulz AP, Thietje R, Matrisch L, Frese J, Hirschfeld S. Incidence of spinal cord injuries in Germany. Eur Spine J. 2023 Feb;32(2):601-7. DOI: 10.1007/s00586-022-07451-0
[4] World Health Organization, editor. International classification of functioning disability and health (ICF). Geneva: WHO; 2001.
[5] ASIA and ISCoS International Standards Committee. The 2019 revision of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)-What's new? Spinal Cord. 2019 Oct;57(10):815-7. DOI: 10.1038/s41393-019-0350-9
[6] Meyer T, Kiekens C, Selb M, Posthumus E, Negrini S. Toward a new definition of rehabilitation for research purposes: a comparative analysis of current definitions. Eur J Phys Rehabil Med. 2020 Oct;56(5):672-81. DOI: 10.23736/S1973-9087.20.06610-1
[7] Mulcahey MJ, Vogel LC, Sheikh M, Arango-Lasprilla JC, Augutis M, Garner E, Hagen EM, Jakeman LB, Kelly E, Martin R, Odenkirchen J, Scheel-Sailer A, Schottler J, Taylor H, Thielen CC, Zebracki K. Recommendations for the National Institute for Neurologic Disorders and Stroke spinal cord injury common data elements for children and youth with SCI. Spinal Cord. 2017 Apr;55(4):331-40. DOI: 10.1038/sc.2016.139
[8] Hodel J, Sabariego C, Galvis Aparicio M, Scheel-Sailer A, Seijas V, Ehrmann C. Revisiting functioning recovery in persons with spinal cord injury undergoing first rehabilitation: Trajectory and network analysis of a Swiss cohort study. PLoS One. 2024 Feb 9;19(2):e0297682. DOI: 10.1371/journal.pone.0297682
[9] Harnett A, Bateman EA, McIntyre A, Parikh R, Middleton J, Arora M, Wolfe D, Mehta S. Spinal Cord Injury Rehabilitation Practices. In: Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Mortenson WB, Hsieh JTC, Noonan VK, Loh E, Sproule S, McIntyre A, Querée M, editors. Spinal Cord Injury Rehabilitation Evidence. SCIRE Professional; 2021. p. 1-126.
[10] Lampart P, Häusler F, Langewitz W, Rubinelli S, Sigrist-Nix D, Scheel-Sailer A. Patients' experiences with goal setting during initial rehabilitation after newly acquired spinal cord injury: A pilot qualitative interview study. J Spinal Cord Med. 2023 Sep;46(5):837-47. DOI: 10.1080/10790268.2022.2095496
[11] Lampart P, Schäppi L, Langewitz WA, Rubinelli S, Sigrist-Nix D, Scheel-Sailer A. Health care professionals' experiences with goal setting during initial rehabilitation after newly acquired spinal cord injury/ disorder - a qualitative focus group study. Front Rehabil Sci. 2022 Aug 18;3:982321. DOI: 10.3389/fresc.2022.982321
[12] Scheel-Sailer A, Post MW, Michel F, Weidmann-Hügle T, Baumann Hölzle R. Patients' views on their decision making during inpatient rehabilitation after newly acquired spinal cord injury-A qualitative interview-based study. Health Expect. 2017 Oct;20(5):1133-42. DOI: 10.1111/hex.12559
[13] Whalley Hammell K. Experience of rehabilitation following spinal cord injury: a meta-synthesis of qualitative findings. Spinal Cord. 2007 Apr;45(4):260-74. DOI: 10.1038/sj.sc.3102034
[14] Nas K, Yazmalar L, Şah V, Aydın A, Öneş K. Rehabilitation of spinal cord injuries. World J Orthop. 2015 Jan 18;6(1):8-16. DOI: 10.5312/wjo.v6.i1.8
[15] Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). AWMF-Regelwerk „Leitlinien“. 2nd ed. Berlin: AWMF; 2020. Available from: https://www.awmf.org/leitlinien/awmf-regelwerk.html
[16] Brignardello-Petersen R, Carrasco-Labra A, Guyatt GH. How to Interpret and Use a Clinical Practice Guideline or Recommendation: Users' Guides to the Medical Literature. JAMA. 2021 Oct 19;326(15):1516-23. DOI: 10.1001/jama.2021.15319
[17] Scheel-Sailer A, Selb M, Gmünder HP, Baumberger M, Curt A, Hund-Georgiadis M, Jordan X, Stucki G; Swiss SCI/D Rehabilitation Interest Group. Towards the implementation of clinical quality management at the national level: description of current types of rehabilitation services for spinal cord injury/disorder in Switzerland using an interdisciplinary consensus process. Eur J Phys Rehabil Med. 2022 Apr;58(2):190-8. DOI: 10.23736/S1973-9087.21.06923-9.
[18] Nishikawa-Pacher A. Research Questions with PICO: A Universal Mnemonic. Publications. 2022;10(3):21. DOI: 10.3390/publications10030021
[19] Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016 Dec 5;5(1):210. DOI: 10.1186/s13643-016-0384-4
[20] Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017 Sep 21;358:j4008. DOI: 10.1136/bmj.j4008
[21] Wells G, Shea B, O'Connell D, Peterson J, Welch V. The Newcastle-Ottawa Scale (NOS) for assessing the quality of case-control studies in meta-analyses. Eur J Epidemiol. 2011;25:603-5.
[22] Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. DOI: 10.1136/bmj.l4898
[23] Mokkink LB, Boers M, van der Vleuten CPM, Bouter LM, Alonso J, Patrick DL, de Vet HCW, Terwee CB. COSMIN Risk of Bias tool to assess the quality of studies on reliability or measurement error of outcome measurement instruments: a Delphi study. BMC Med Res Methodol. 2020 Dec 3;20(1):293. DOI: 10.1186/s12874-020-01179-5
[24] OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence. Oxford: Oxford Centre for Evidence-Based Medicine; 2011.
[25] Deutschsprachige Medizinische Gesellschaft für Paraplegiologie e.V., et al. S2e-Leitlinie Ergebniserhebung in der Erstbehandlung nach neu erworbener Querschnittlähmung. Version 1.0. AWMF-Registernummer 179 - 012. Berlin: AWMF; 2020. Available from: https://register.awmf.org/de/leitlinien/detail/179-012
[26] Masuhr K, Masuhr F, Neumann M. Neurologie. 7th ed. Stuttgart, New York: Thieme; 2013. (Duale Reihe).
[27] Niethard FU, Pfeil J, Biberthaler P. Orthopädie und Unfallchirurgie. 8th ed. Stuttgart: Thieme; 2017. (Duale Reihe).
[28] Hislop HJ, Montgomery J, editors. Manuelle Muskeltests: Untersuchungstechniken nach Daniels und Worthingham. 8th edl. München, Jena: Elsevier, Urban & Fischer; 2007.
[29] Schädler S, Kool J, Lüthi H, Marks D, Oesch P, Pfeffer A, Wirz M, editors. Assessments in der Rehabilitation. 4th ed. Göttingen: Hogrefe; 2019.
[30] Smolenski UC, Buchmann J, Beyer L , Harke G, Pahnke J, Seidel W. Janda Manuelle Muskelfunktionsdiagnostik: Theorie und Praxis. 5th ed. München: Urban & Fischer; 2016.
[31] Deutschsprachige Medizinische Gesellschaft für Paraplegiologie e.V., et al. S2k-Leitlinie Schmerzen bei Querschnittlähmung. Version 1.0. AWMF-Registernummer: 179/006. Berlin: AWMF; 2018.
[32] EMSCI. Time Schedule: European Multicenter Study about Spinal Cord Injury. 2023. Available from: https://www.emsci.org/index.php/project/the-project/time-schedule
[33] ;Deutschsprachige Medizinische Gesellschaft für Paraplegiologie e.V., et al. S2e-Leitlinie Verbesserung der Funktionsfähigkeit der oberen Extremität bei zervikaler Querschnittlähmung. AWMF-Registernummer (179-013). Berlin: AWMF; 2020.
[34] Steiner WA, Ryser L, Huber E, Uebelhart D, Aeschlimann A, Stucki G. Use of the ICF model as a clinical problem-solving tool in physical therapy and rehabilitation medicine. Phys Ther. 2002 Nov;82(11):1098-107.
[35] Hornby TG, Reisman DS, Ward IG, Scheets PL, Miller A, Haddad D, Fox EJ, Fritz NE, Hawkins K, Henderson CE, Hendron KL, Holleran CL, Lynskey JE, Walter A; and the Locomotor CPG Appraisal Team. Clinical Practice Guideline to Improve Locomotor Function Following Chronic Stroke, Incomplete Spinal Cord Injury, and Brain Injury. J Neurol Phys Ther. 2020 Jan;44(1):49-100. DOI: 10.1097/NPT.0000000000000303
[36] Clinicians SCICPL. Clinical Guideline for Standing Adults following Spinal Cord Injury. MASCIP; 2019.
[37] Deutschsprachige Medizinische Gesellschaft für Paraplegiologie e.V. (DMGP), et al.. S2e-Leitlinie Rehabilitation der unteren Extremität, der Steh- und Gehfunktion bei Menschen mit Querschnittlähmung. Version 2.0. Registernummer 179-009. Berlin: AWMF; 2024. Available from: https://register.awmf.org/de/leitlinien/detail/179-009
[38] Tomaschek R, Gemperli A, Rupp R, Geng V, Scheel-Sailer A; German-speaking Medical SCI Society (DMGP) Ergebniserhebung Guideline Development Group. A systematic review of outcome measures in initial rehabilitation of individuals with newly acquired spinal cord injury: providing evidence for clinical practice guidelines. Eur J Phys Rehabil Med. 2019 Oct;55(5):605-17. DOI: 10.23736/S1973-9087.19.05676-4
[39] Poncumhak P, Saengsuwan J, Kamruecha W, Amatachaya S. Reliability and validity of three functional tests in ambulatory patients with spinal cord injury. Spinal Cord. 2013 Mar;51(3):214-7. DOI: 10.1038/sc.2012.126
[40] van Hedel HJ, Wirz M, Dietz V. Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests. Arch Phys Med Rehabil. 2005 Feb;86(2):190-6. DOI: 10.1016/j.apmr.2004.02.010
[41] Amatachaya S, Naewla S, Srisim K, Arrayawichanon P, Siritaratiwat W. Concurrent validity of the 10-meter walk test as compared with the 6-minute walk test in patients with spinal cord injury at various levels of ability. Spinal Cord. 2014 Apr;52(4):333-6. DOI: 10.1038/sc.2013.171
[42] Willi R, Widmer M, Merz N, Bastiaenen CHG, Zörner B, Bolliger M. Validity and reliability of the 2-minute walk test in individuals with spinal cord injury. Spinal Cord. 2023 Jan;61(1):15-21. DOI: 10.1038/s41393-022-00847-1
[43] Aigner A, Curt A, Tanadini LG, Maathuis MH. Concurrent validity of single and groups of walking assessments following acute spinal cord injury. Spinal Cord. 2017 May;55(5):435-40. DOI: 10.1038/sc.2016.148
[44] Abou L, Ilha J, Romanini F, Rice LA. Do clinical balance measures have the ability to predict falls among ambulatory individuals with spinal cord injury? A systematic review and meta-analysis. Spinal Cord. 2019 Dec;57(12):1001-13. DOI: 10.1038/s41393-019-0346-5
[45] Arora T, Oates A, Lynd K, Musselman KE. Current state of balance assessment during transferring, sitting, standing and walking activities for the spinal cord injured population: A systematic review. J Spinal Cord Med. 2020 Jan;43(1):10-23. DOI: 10.1080/10790268.2018.1481692
[46] Arsh A, Darain H, Ullah I, Shakil-Ur-Rehman S. Diagnostic tests to assess balance in patients with spinal cord injury: a systematic review of their validity and reliability. Asian Biomed (Res Rev News). 2021 Jun 30;15(3):111-8. DOI: 10.2478/abm-2021-0014
[47] Jørgensen V, Opheim A, Halvarsson A, Franzén E, Roaldsen KS. Comparison of the Berg Balance Scale and the Mini-BESTest for Assessing Balance in Ambulatory People With Spinal Cord Injury: Validation Study. Phys Ther. 2017 Jun 1;97(6):677-87. DOI: 10.1093/ptj/pzx030
[48] Lemay JF, Nadeau S. Standing balance assessment in ASIA D paraplegic and tetraplegic participants: concurrent validity of the Berg Balance Scale. Spinal Cord. 2010 Mar;48(3):245-50. DOI: 10.1038/sc.2009.119
[49] Chan K, Unger J, Lee JW, Johnston G, Constand M, Masani K, Musselman KE. Quantifying balance control after spinal cord injury: Reliability and validity of the mini-BESTest. J Spinal Cord Med. 2019 Oct;42(sup1):141-8. DOI: 10.1080/10790268.2019.1647930
[50] Roy A, Higgins J, Nadeau S. Reliability and minimal detectable change of the mini-BESTest in adults with spinal cord injury in a rehabilitation setting. Physiother Theory Pract. 2021 Jan;37(1):126-34. DOI: 10.1080/09593985.2019.1622161
[51] Arsh A, Darain H, Rahman MU, Ullah I, Shakil-Ur-Rehman S. Reliability of modified functional reach test in the assessment of balance function in people with spinal cord injury: A systematic review. J Pak Med Assoc. 2021 Aug;71(8):2040-44. DOI: 10.47391/JPMA.1276
[52] Mills PB, Vakil AP, Phillips C, Kei L, Kwon BK. Intra-rater and inter-rater reliability of the Penn Spasm Frequency Scale in People with chronic traumatic spinal cord injury. Spinal Cord. 2018 Jun;56(6):569-74. DOI: 10.1038/s41393-018-0063-5
[53] Akpinar P, Atici A, Ozkan FU, Aktas I, Kulcu DG, Kurt KN. Reliability of the Spinal Cord Assessment Tool for Spastic Reflexes. Arch Phys Med Rehabil. 2017 Jun;98(6):1113-8. DOI: 10.1016/j.apmr.2016.09.119
[54] Ellapen TJ, Hammill HV, Swanepoel M, Strydom GL. The benefits of hydrotherapy to patients with spinal cord injuries. Afr J Disabil. 2018 May 16;7(0):450. DOI: 10.4102/ajod.v7i0.450
[55] Li C, Khoo S, Adnan A. Effects of aquatic exercise on physical function and fitness among people with spinal cord injury: A systematic review. Medicine (Baltimore). 2017 Mar;96(11):e6328. DOI: 10.1097/MD.0000000000006328
[56] Amatachaya S, Srisim K, Arrayawichanon P, Thaweewannakij T, Amatachaya P. Dual-Task Obstacle Crossing Training Could Immediately Improve Ability to Control a Complex Motor Task and Cognitive Activity in Chronic Ambulatory Individuals With Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2019 Summer;25(3):260-70. DOI: 10.1310/sci18-00038
[57] Fang CY, Lien AS, Tsai JL, Yang HC, Chan HL, Chen RS, Chang YJ. The Effect and Dose-Response of Functional Electrical Stimulation Cycling Training on Spasticity in Individuals With Spinal Cord Injury: A Systematic Review With Meta-Analysis. Front Physiol. 2021 Nov 19;12:756200. DOI: 10.3389/fphys.2021.756200
[58] van der Scheer JW, Goosey-Tolfrey VL, Valentino SE, Davis GM, Ho CH. Functional electrical stimulation cycling exercise after spinal cord injury: a systematic review of health and fitness-related outcomes. J Neuroeng Rehabil. 2021 Jun 12;18(1):99. DOI: 10.1186/s12984-021-00882-8
[59] Ibitoye MO, Hamzaid NA, Hayashibe M, Hasnan N, Davis GM. Restoring prolonged standing via functional electrical stimulation after spinal cord injury: A systematic review of control strategies. Biomedical Signal Processing and Control. 2019;49:34-47. DOI: 10.1016/j.bspc.2018.11.006
[60] Ben M, Harvey L, Denis S, Glinsky J, Goehl G, Chee S, Herbert RD. Does 12 weeks of regular standing prevent loss of ankle mobility and bone mineral density in people with recent spinal cord injuries? Aust J Physiother. 2005;51(4):251-6. DOI: 10.1016/s0004-9514(05)70006-4
[61] Adams MM, Hicks AL. Comparison of the effects of body-weight-supported treadmill training and tilt-table standing on spasticity in individuals with chronic spinal cord injury. J Spinal Cord Med. 2011;34(5):488-94. DOI: 10.1179/2045772311Y.0000000028
[62] Stampacchia G, Gazzotti V, Olivieri M, Andrenelli E, Bonaiuti D, Calabro RS, Carmignano SM, Cassio A, Fundaro C, Companini I, Mazzoli D, Cerulli S, Chisari C, Colombo V, Dalise S, Mazzoleni D, Melegari C, Merlo A, Boldrini P, Mazzoleni S, Posteraro F, Mazzucchelli M, Benanti P, Castelli E, Draicchio F, Falabella V, Galeri S, Gimigliano F, Grigioni M, Mazzon S, Molteni F, Morone G, Petrarca M, Picelli A, Senatore M, Turchetti G, Bizzarrini E. Gait robot-assisted rehabilitation in persons with spinal cord injury: A scoping review. NeuroRehabilitation. 2022;51(4):609-47. DOI: 10.3233/NRE-220061
[63] Mehrholz J, Harvey LA, Thomas S, Elsner B. Is body-weight-supported treadmill training or robotic-assisted gait training superior to overground gait training and other forms of physiotherapy in people with spinal cord injury? A systematic review. Spinal Cord. 2017 Aug;55(8):722-9. DOI: 10.1038/sc.2017.31
[64] Gaspar R, Padula N, Freitas TB, de Oliveira JPJ, Torriani-Pasin C. Physical Exercise for Individuals With Spinal Cord Injury: Systematic Review Based on the International Classification of Functioning, Disability, and Health. J Sport Rehabil. 2019 Jul 1;28(5):505-16. DOI: 10.1123/jsr.2017-0185
[65] Lawrason SVC, Todd KR, Shaw RB, Martin Ginis KA. Physical activity among individuals with spinal cord injury who ambulate: a systematic scoping review. Spinal Cord. 2020 Jul;58(7):735-45. DOI: 10.1038/s41393-020-0460-4
[66] Quel de Oliveira C, Refshauge K, Middleton J, de Jong L, Davis GM. Effects of Activity-Based Therapy Interventions on Mobility, Independence, and Quality of Life for People with Spinal Cord Injuries: A Systematic Review and Meta-Analysis. J Neurotrauma. 2017 May 1;34(9):1726-43. DOI: 10.1089/neu.2016.4558
[67] Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. DOI: 10.1136/bmj.n71
Attachments
| Attachment 1 | Supplementary material (000338_Attachment1.pdf, application/pdf, 5.08 MBytes) |