Internal body measurements and composition in calves
Albert Brun 1
Marta Terré 2
1 Food Quality and Technology Program, IRTA, Monells, Girona, Spain
2 Ruminant Production, IRTA, Caldes de Montbui, Spain
Introduction
Techniques to estimate body composition of growing animals are necessary to refine estimates of nutrient requirements in young animals of different breeds (e.g., crossbreed, Holstein, Angus), which reach physiological maturity at different body weights and ages. This is important as it allows for adjustment of the nutrient composition of diets, and is useful for evaluating different feeding and management strategies. Traditionally, body composition was evaluated by slaughtering animals at different ages [1], [2]. However, the development of non-invasive techniques to estimate body composition are essential to allow for adjustment of nutrient requirements in young animals, reducing the number of animals needed and refining the methodology for body composition estimation.
Computed tomography (CT) is a non-invasive technology based on X-rays, where rays are attenuated as they move through the body. The degree of attenuation is measured with detectors and then an image of the internal body is created in grey scale via reconstruction algorithms. The intensity of grey is related to the density of the tissue. It is possible to differentiate adipose, muscular and bone tissues because their density is different. From the images created, it is possible to obtain linear measurements and cross-sectional areas and, thereby, to quantify the amount of each tissue [3], [4], [5]. CT has been used in livestock, mainly in pigs and lambs to measure their composition at a specific stage of growth for breeding purposes or body characterization and at different stages of growth, to develop growth curves [4], [6], [7]. In calves, CT has been used to evaluate the fat and lean content of the carcass, and white and red viscera in animals fed differing levels of milk replacer [5] or amino acid supplementation [8].
Dual X-ray absorptiometry (DXA) and magnetic resonance imaging (MRI) are also non-invasive technologies suitable for evaluating body characteristics of calves [9]. DXA is based on X-rays combining a high and a low energy level and has lower radiation than CT. DXA allows users to measure body composition, but it does not provide an image of the internal body of the animal. It has been used to determine bone mineralization and body composition in calves [10]. MRI is based on magnetic field and has the advantage that it does not produce irradiation. However, it is much slower than CT. MRI also creates an image of the interior of the animal, allowing areas and thicknesses to be calculated, as well as body composition. It has been used to evaluate visceral adipose tissue volume of calves [11].
Prerequisites
The CT device has some technically based requirements, which must be met by the animals to be scanned, mainly related to their weight and maximum width. The weight that can be supported by the CT table is limited and although it differs with devices, in a medical device it may be around 200 kg. Thus, this sets limits to the weight of the animal that can be scanned. Furthermore, the gantry has a diameter of around 75 cm. The displayed field of view (DFOV) is lower, at around 50 cm, which is the maximum width the device can scan. In medical scanners, this could be the most important limitation for calves, rather than weight, as animals of less than 100 kg can have a hip width of around 50 cm. However, there are veterinary devices that allow scanning of larger animals, avoiding these limitations. With CT, it is possible to measure carcass quality, detailed in the Animal Train Ontology for Livestock (ATOL) number ATOL_0000090 (for complete list of ATOL, please visit https://www.atol-ontology.com/en/erter-2).
A – Computed tomography (CT) scanning
- Before starting daily, the CT scanner needs to warm up (45–60 minutes) and then a daily calibration protocol must be applied.
- The scanning protocol should be established depending on the objective of the measurement and the experience of the group, defining the matrix size, the kV and mA applied, the thickness of the measure and the frequency of each measure, the collimation, and all the other technical parameters.
- Calves must be sedated, for example with an intramuscular injection of xylazine at a dose of 0.01 mL/kg body weight.
- Since the DFOV is a circle, it is recommended to place the animal in a tube (Figure 1) or to keep the diameter fixed with a soft cover to avoid exceeding the maximum diameter.
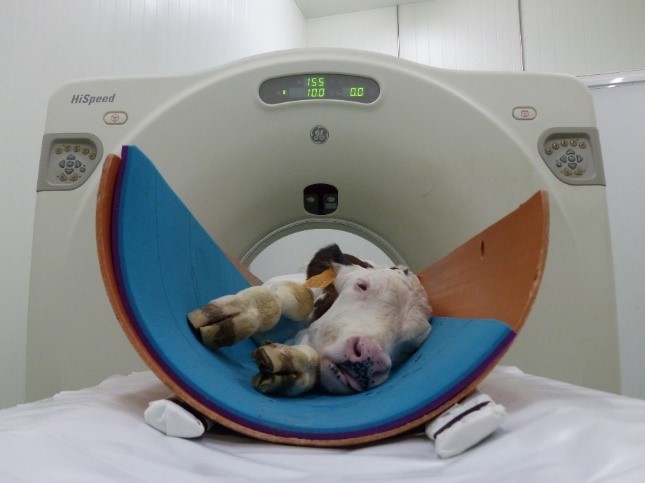
Figure 1: Scanning of a live calf, placed in a polystyrene tube, with computed tomography (CT) equipment - The height of the table should be set to centre the calf on the gantry as far as is possible. At the start of scanning, the table should be moved to define the initial coordinates.
- After the identification of the animal (and the project) in the records, the protocol can be applied, and the scanning is performed. Once finished, images must be saved for further processing.
B – Basics of computed tomography (CT) analysis
- CT measurements are based on attenuation of X-rays on their way through the body, producing a 3D image. The image is composed by voxels (3D pixels), whose size (volume) is determined by the matrix, the DFOV and the thickness. Each voxel has an attenuation value that is measured in Hounsfield units (HU).
- In the HU scale, water has a HU of 0 and air of –1.000. Negative values indicate density lower than water, whilst positive values indicate a density higher than water. Fat has HU values from 0 to –120/–140. Muscle has HU values from 0 to +120/+140. Bones have HU values higher than +140. In a grey scale, lower HU values are darker than higher HU values, thus bones are lighter than lean, and lean is lighter than fat. Air is black. The window (width and position) of the grey scale can be adjusted to better visualize the tissues of interest (Figure 2). In CT images it is possible to find the partial volume effect, i.e. voxels that are not 100% one tissue but a mixture of several tissues (e.g. lean and fat, or lean and bone), and because of that, sometimes they are difficult to classify and segmentation techniques are necessary.
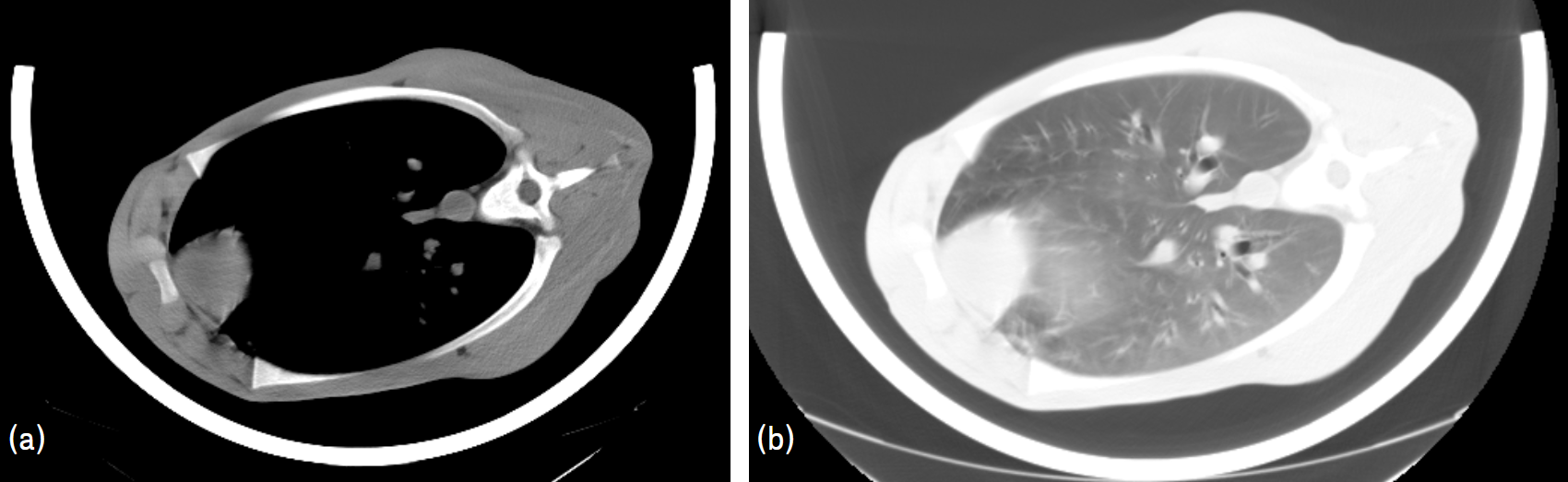
Figure 2: Image of the thoracic region of a calf with a window to visualize the lean, muscle and bones (a) and, the same image, with a window to visualize the lungs (b)
C – Computed tomography (CT) image analysis
- From CT images it is possible to take direct measurements in a specific anatomical position, either lineal (thickness, width, height), angles or surfaces (area) (Figure 3). This allows for measurement of organ size and tissue thickness or area size.
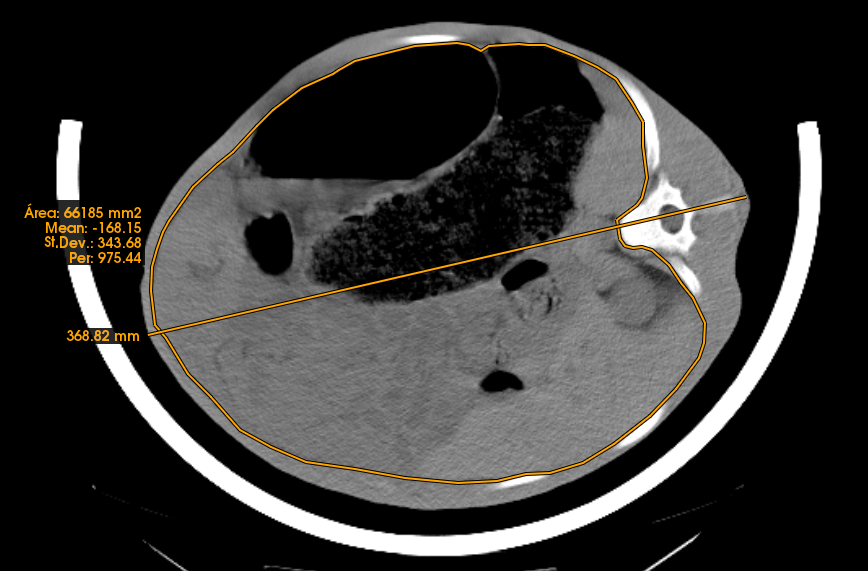
Figure 3: Lineal and area measurements in the abdominal region - The volume associated with each voxel can be determined from each image obtained from one animal and a histogram can be plotted (Figure 4). Usually, the histogram has a peak for fat and a peak for lean (see examples of finishing pigs in Figure 4b). In between the two peaks, there are voxels with partial volume effect. In calves, the fat tissue is not fully developed and therefore it is not possible to find many voxels, which are pure fat tissue. Thus, the partial volume effect is important and due to this effect and the lack of fat, the peak for fat does not exist. However, for calves, the lower part of the lean curve varies in shape dependent on the fat content, and this can be used to obtain a calibration equation to determine it (see Font-i-Furnols et al., 2021 [5]). The volume can be transformed to weight by applying a density.
- From the histograms, it is possible to obtain calibration equations to estimate the fat and lean content. These equations need to be validated if other types of animals are used. Additionally, if another CT scanner is used or a different scanning protocol is applied the equations must be validated.
- When the target of the study is bone characteristics, linear or area measurements of the full bone or the cortical region of the bone can be computed. Additionally, the volume associated with each Hounsfield unit can be determined and bone density calculated. When bone density could be affected by the diet or other factors, it might be of interest to determine the proportion of bones with low, medium, and high density.
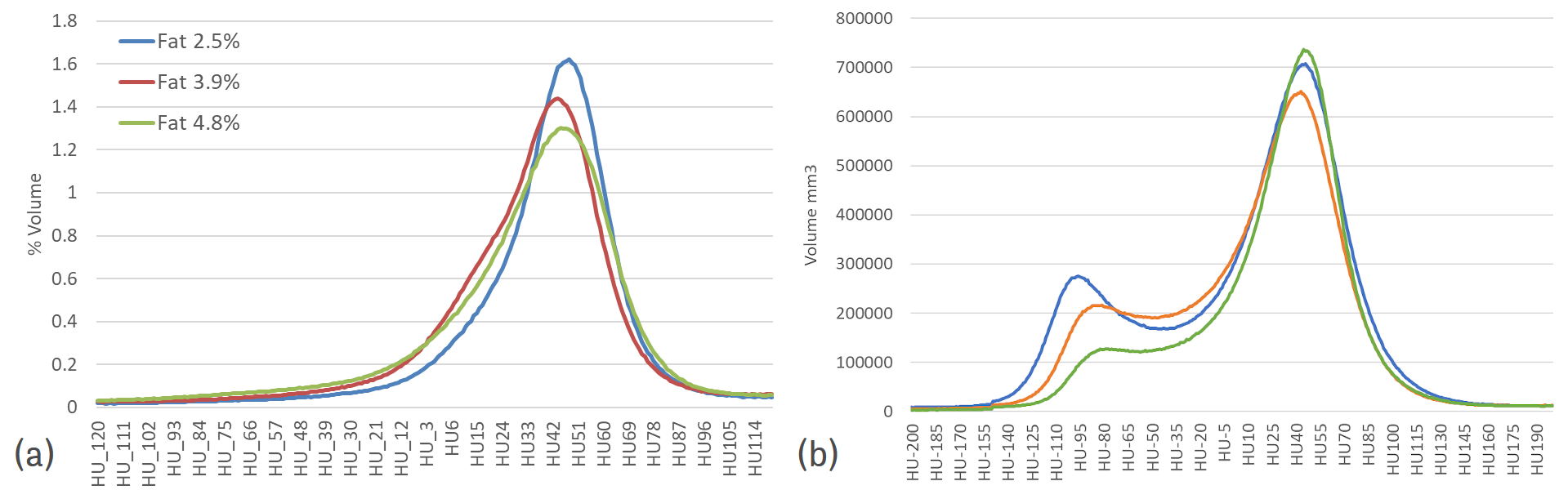
Figure 4: Distribution of the volume associated to Hounsfield units of the images obtained from (a) five calves with different fat levels, and (b) three finishing pigs with 3 different levels of fatness
References
[1] Bartlett KS, McKeith FK, VandeHaar MJ, Dahl GE, Drackley JK. Growth and body composition of dairy calves fed milk replacers containing different amounts of protein at two feeding rates. J Anim Sci. 2006 Jun;84(6):1454-67. DOI: 10.2527/2006.8461454x[2] Diaz MC, Van Amburgh ME, Smith JM, Kelsey JM, Hutten EL. Composition of Growth of Holstein Calves Fed Milk Replacer from Birth to 105-Kilogram Body Weight. J Dairy Sci. 2001 Apr;84(4):830-42. DOI: 10.3168/jds.S0022-0302(01)74541-9
[3] Font-i-Furnols M, Brun A, Martí S, Realini CE, Pérez-Juan M, Gonzalez J, Devant M. Composition and intramuscular fat estimation of Holstein bull and steer rib sections by using one or more computed tomography cross-sectional images. Livest Sci. 2014 Dec;170:210-8. DOI: 10.1016/j.livsci.2014.10.009
[4] Carabús A, Sainz RD, Oltjen JW, Gispert M, Font-i-Furnols M. Predicting fat, lean and the weights of primal cuts for growing pigs of different genotypes and sexes using computed tomography. J Anim Sci. 2015 Mar;93(3):1388-97. DOI: 10.2527/jas.2014-8697
[5] Font-i-Furnols M, Terré M, Brun A, Vidal M, Bach A. Prediction of tissue composition of live dairy calves and carcasses by computed tomography. Livest Sci. 2021 Jan;243:104371. DOI: 10.1016/j.livsci.2020.104371
[6] Lambe NR, Bünger L, Bishop SC, Simm G, Conington J. The effect of selection indices for sustainable hill sheep production on carcass composition and muscularity of lambs, measured using X-ray computed tomography. Animal. 2008;2(1):27-35. DOI: 10.1017/S1751731107000924
[7] Carabús A, Sainz RD, Oltjen JW, Gispert M, Font-I-Furnols M. Growth of total fat and lean and of primal cuts is affected by the sex type. Animal. 2017 Aug;11(8):1321-29. DOI: 10.1017/S1751731117000039
[8] Terré M, Font-Furnols M, Bassols A, Vidal M, Brun A, Bach A. Amino acid supplementation in calf milk replacer. In: Annual Meeting of the American Dairy Science Association (ADSA); 2018 Jun 24-27; Knoxville, TN, USA.
[9] Danesh Mesgaran S, Pomies D, Röttgen V, Kuhla B, Nüske S, Bach A, Scholz AM. Milk intake, body anatomy and composition in calves. In: Mesgaran SD, Baumont R, Munksgaard L, Humphries D, Kennedy E, Dijkstra J, Dewhurst R, Ferguson H, Terré M, Kuhla B, editors. Methods in cattle physiology and behaviour - Recommendations from the SmartCow consortium. Cologne: PUBLISSO; 2020-. DOI: 10.5680/mcpb009
[10] Scholz AM, Nüske S, Förster M. Body composition and bone mineralization measurement in calves of different genetic origin by using dual-energy X-ray absorptiometry. Acta Diabetol. 2003 Oct;40 Suppl 1:S91-4. DOI: 10.1007/s00592-003-0037-7
[11] Kappes R, Schneider V, Schweizer H, Nüske S, Knob DA, Thaler Neto A, Scholz AM. Effect of β-casein A1 or A2 milk on body composition, milk intake, and growth in Holstein, Simmental, and crossbred dairy calves of both sexes. J Dairy Sci. 2024 Jun;107(6):4033-44. DOI: 10.3168/jds.2023-24046




