Non-antimicrobial prophylactic measures in recurrent urinary tract infections
Franck Bruyère 3,4
1 Urology, Poitiers University Hospital, Poitiers, France
2 INSERM U1070, Pharmacologie des Anti-infectieux; UFR Médecine-Pharmacie, Université de Poitiers; Poitiers; France
3 Urology, CHRU Tours, France
4 Université François Rabelais de Tours, France
Abstract
The epidemiology of rUTIs is, at present, uncertain. About 50% of women will have UTI in their lives at least once, and 20% to 30% of these women will suffer again at least once. However, the proportion of these women with real rUTIs, at least 3 to 4 episodes per year, is not clearly known. The importance of the impact on the quality of life of women with rUTIs, as well as the impact of an increase in antibiotic prescriptions and bacterial resistance, have made this pathology an important area of research for many years now. The effectiveness of long-term antibiotic prophylaxis is demonstrated, however, the need to find alternatives to antibiotic treatment is essential today. The aim of this study was to conduct a systematic review of the literature on non-antimicrobial prophylactic measures available to prevent recurrence in adult women with rUTIs.
A systematic review of the literature was performed according to the criteria PRISMA. All national and international recommendations were reviewed. A systematic literature search was performed for the last 20 years in the Cochrane and Medline libraries on 2018-07-09. The criteria for the studies to be retained were: all studies concerning female patients over 18 years who present rUTIs and received a different treatment than antibiotibic. After exclusion of duplicates a total of 226 publications were identified. A total of 70 publications were assessed for eligibility. In total, 34 studies were included into the review analysis.
Cranberry, immunoactive prophylaxis, and vaginal oestrogen replacement in postmenopausal women are measures that can be recommended for the prevention of rUTIs in women. On the other hand, other measures such as probiotics, bacterial interference, endovesical instillation, behavioural modifications, Chinese herbal medicine, acupuncture, or D-mannose are not measures that can be currently recommended for the prevention of rUTIs.
The challenge is twofold: to improve the quality of life of these patients, while limiting the prescription of antibiotics in the long term. Unfortunately, many of these non-antibiotic measures turn out to be alternatives that are often less effective than antibiotics, which remains the reference.
The paradox of this pathology is to propose as many therapeutic alternatives for a pathology without risk but source of many difficulties for the patients as well as the practitioners who take care of them. Finally, recent work on urinary microbiota appears to be an essential step in understanding this complex pathology whose physiopathology is still uncertain.
Summary of Recommendations
-
Given its good tolerance both in the short term and in the long term, cranberry at a dosage of at least 36 mg/day seems to be recommended and tried as an adjunctive measure for the prevention of rUTIs in women who are demanding and/or in failure of other measures (GoR C, LoE 4).
-
The use of immunoactive prophylaxis is recommended (GoR A, LoE 1a) given its effectiveness and excellent tolerance. In view of the better quality studies available and the greater decline in its use, Uro-Vaxom® is recommended as first line. However, Urovac® may also be prescribed as first-line therapy if the patient wishes, due to an administrative method that requires less long-term observance.
-
Given its good tolerability and ease of use, vaginal oestrogen replacement may be recommended (GoR A; LoE 1b) for the prevention of rUTIs but only in postmenopausal women.
-
Although behavioural modifications (post-coital urination, increasing fluid intake, wiping from front to back after defecation, wearing cotton underwear and reduced douching) are safe, some may appear to be restrictive for some patients and there is no reason to recommend them in the current state of knowledge (GoR B, LoE 2a). Nevertheless, a thorough interrogation will make it possible to identify the patients with bad habits whose implication in the genesis of rUTIs appears obvious and to whom these measures can be recommended.
1 Introduction
The lack of severity of recurrent urinary tract infections (rUTIs) often contrasts with the importance of the impact on the quality of life of women affected by this disease [1], [2], [3]. Indeed, the fate of cystitis, except for particular clinical situations, is very rarely pyelonephritis [4], [5], and the issue of management of recurrent cystitis is therefore not in preventing a possible complication of this pathology but in improving the quality of life of these women.
The epidemiology of rUTIs is, at present, uncertain. In the United States, the incidence of UTIs is estimated at 12.6% of women over 18 [6]. About 50% of women will have UTI in their lives at least once, and 20% to 30% of these women will suffer again at least once [1]. However, the proportion of these women with real rUTIs, at least 3 to 4 episodes per year [7], [8], is not clearly known.
The importance of the impact on the quality of life of women with rUTIs, as well as the impact of an increase in antibiotic prescriptions and bacterial resistance [9], [10], have made this pathology an important area of research for many years now.
Physiopathology, in light of discoveries in recent years regarding the urinary microbiota [11], does not seem entirely established today which probably explains both the difficulty that clinicians sometimes encounter in treating these patients but also the vast arsenal of therapy that is proposed today to manage this pathology.
The effectiveness of long-term antibiotic prophylaxis is demonstrated [7], [8], however, the need to find alternatives to antibiotic treatment is essential today, especially as a means of saving the use of these antibiotics whose future is now threatened [9]. Nevertheless, there are many alternatives to antibiotic prophylaxis, but their roles in the treatment strategy of these patients is not always clear.
The aim of this study was to conduct a systematic review of the literature on non-antimicrobial prophylactic measures available to prevent recurrence in adult women with rUTIs.
2 Methods
This systematic review of the literature was performed according to the criteria of Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) [12].
Eligibility criteria:
The criteria for the studies to be retained were: all studies concerning female patients over 18 years who present rUTIs and have received treatments which are different from antibioprophylaxis.
Search Strategy:
All national and international recommendations were reviewed. A systematic literature search was performed for the last 20 years in the Cochrane and Medline libraries on 2018-07-09 using the following MeSH keywords: adult women, antibiotic, antibioprophylaxis, bacterial interference, cranberry, cystitis, diet therapy, drug therapy, oestrogens, Lactobacillus, lower urinary tract infection, proanthocyanidin, prevention and control, probiotic, recurrent urinary tract infections, surgery, therapy and vaccines. The following limitations were: adults, humans, abstract available, French and English languages. The following search algorithm was used: (Recurrent) AND (Urinary tract infections OR lower urinary tract infection OR cystitis) AND (Adult women) AND (Oestrogens OR Lactobacillus OR Cranberry OR proanthocyanidin OR Probiotic OR diet therapy OR drug therapy OR vaccines OR bacterial interference OR prevention and control OR surgery OR therapy) NOT Antibiotic NOT Antibiotic prophylaxis. The articles were selected according to their methodology, language (English/French), and relevance to this study. An Internet search also identified other references, including guidelines.
Study selection and data extraction:
All articles were selected and audited by two authors. All the studies collected, whether retrospective or prospective, were analysed. Only data related to studies linking non-antimicrobial prevention or treatment of rUTIs were collected.
Studies whose abstract was not available were excluded from the outset, as were basic research studies. Other reasons for exclusions included: obsolete data, studies with low level of evidence, studies with wrong methodology, endpoint not relevant, full text unavailable, and studies already included in meta-analysis.
The selection of studies is summarized in figure 1. After exclusion of duplicates a total of 226 publications were identified, which were screened by title and abstract. A total of 70 publications were assessed for eligibility. In total, 34 studies were included into the review analysis. Data collection was then done liberally by M.V., followed by verification by F.B.
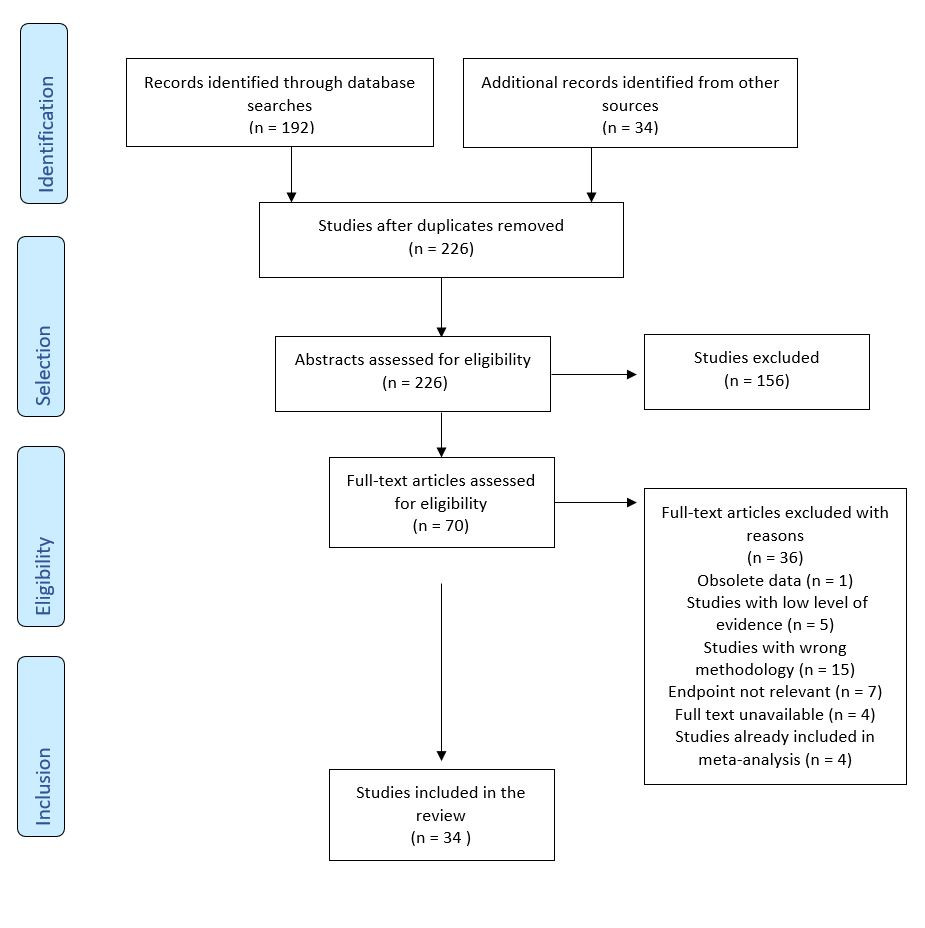
3 Results
3.1 Cranberry
Cranberry has been widely studied in the medical field for the last twenty years, particularly in the prevention of recurrent cystitis. The difficulty when approaching the cranberry is to know what we are talking about precisely. Indeed, there are more than 400 different species of this plant of the genus Vaccinium. When we talk about cranberry, we actually talk about 4 species: V. macrocarpon, V. vitis idaea, V. oxycocus, and V. eruthrocarpum [13]. V. macrocarpon has been the most studied and seems to be the only one to have a real therapeutic effectiveness. Whatever the species considered, it is also important to differentiate between cranberry juice and the active ingredient used in drugs with very different properties.
Cranberry is composed of 4 main components: organic acids, flavonoids, iridoid glycosides and finally anthocyanidins [13]. Among the anthocyanidins, proanthocyanidin (PAC) type A2 is the molecule having properties limiting the adhesion of bacteria to the urothelium and in particular E. coli. In fact, PAC will inhibit the synthesis of P-fimbriae and E. coli type I pili and therefore the adhesins that constitute the "anchor point" between the bacteria and the urothelial D-mannose and polysaccharide receptors [13], [14], [15]. V. myrtillus, commonly called blueberry, also contains proanthocyanidins but to a lesser extent which is why we will focus on talking only about cranberry in this section [13].
Current guidelines vary according to learned societies. The EAU has not issued a recommendation for contradictory trials and therefore takes no position for or against the use of cranberry [8]. Canadian associations of urology and gynecology (CUA and SOGC) recommend cranberry products (no distinction is made between juices and drugs), however, these guidelines are old and not based on the most recent data [16], [17]. The American and English guidelines do not evoke this means of prevention and do not present any recommendations [18], [19]. Finally, the French guidelines recommend the prescription of a PAC active ingredient of 36 mg/day with a low level of evidence (GoR C, LoE 4) [7].
Taking into account only the publications of good methodological quality, we retain only the Cochrane review by Jepson et al. [20]. This shows the difficulty of proving the effectiveness of cranberry as the active ingredient, the dosage forms and the concentrations evaluated are different from one study to another. This review brings together a total of 24 studies and 4473 participants, despite the Cochrane methodology, overall heterogeneity was high (I2 = 55%), which again shows the difficulty of truly concluding the effectiveness of cranberry. The conclusion of this review is that cranberry juice does not seem to have superiority compared to a placebo for the prevention of rUTIs. It should be noted, however, that this is a 4th update of this Cochrane review, the first of which was published in 1998 by the same authors. The conclusion of this 1998 review was that there was no sufficient scientific evidence to prove the effectiveness of cranberry in preventing rUTIs [21]. The authors' conclusion was different in 2004 and suggested, based on two new randomised trials, that cranberry appeared to be effective but that the ideal dosage and route of administration remained to be defined [22]. In 2008, the update of this review confirmed the results of 2004 [23], before it became negative again in 2012. This could partly be explained by the addition of certain studies to the questionable methodology with a high risk of bias, which again proves the great disparity of protocols in studies using cranberry. No high quality methodological trial has been published since this Cochrane review.
Nevertheless, given its good tolerance both in the short term and in the long term [13], Cranberry at a dosage of at least 36 mg/day seems to be recommendable and tried as an adjunctive measure for the prevention of rUTIs in women who are demanding and/or in failure of other measures (GoR C, LoE 4). Nevertheless, it will be necessary to ensure that there is no risk of drug interactions, particularly with oral anticoagulants due to the interaction of cranberry with CYP3A4 cytochrome [13].
A recent in-vitro study using high doses of cranberry PAC (190 mg/L) combined with propolis (102.4 mg/L) showed a significant reduction in adhesion, motility, biofilm formation and of UPEC metabolism [24]. A study soon to be published has compared a population of homogenous women with rUTIs using the combination of propolis-cranberry (DUAB) or a placebo. The conclusions of this randomised multicenter study were that the benefit of cranberry, to reduce the number of UTIs, is effective but only in the first 3 months of treatment (CysDUA Study). There seemed to be some habituation making the analysis negative at 6 months.
These results must of course be confirmed but this suggests that the undeniable success of cranberry in the prevention of rUTIs may be when in combination with other molecules and not alone.
3.2 Immunoactive prophylaxis
The vaccine concept in UTI prevention is old and dates back to 1956 [25]. Immunisation for the prevention of UTIs presents many pitfalls: the great diversity of pathogenic bacterial species as well as the great diversity within a pathogenic bacterial type and finally the difficulty for the immune system to keep a lasting and sufficient immunological imprint and to protect the urothelium from a pathogenic bacterial species [15]. The physiopathology of a UTI is complex and still not fully understood, which also partly explains the difficulty of finding a vaccine that would be effective in a sustainable manner. Currently, therefore, the term "immunoactive prophylaxis" should be preferred to "vaccine". Indeed, the current principle is to expose patients repeatedly to different attenuated pathogenic bacteria serotypes in order to stimulate the immune system via the urothelial MALT (mucosa associated lymphoid tissue) in order to promote the recognition of pathogenic bacterial antigens, notably via stimulation of dentritic cells and an increased local production of IgA and IgG.
First of all, it is important to acknowledge all of the different types of vaccines available on the market:
-
Oral or sublingual vaccines: OM89 (Uro-Vaxom®), Urvakol® and Urostim®, Uromune®
-
Intramuscular vaccines: StroVac®, FimCH, ExPEC4V
-
Vaccines administered intravaginally: SolcoUrovac®
Each of these vaccines has a different composition with more or less bacteria species. Some are composed only of pathogenic strains of E. coli (Uro-Vaxon®, ExPEC4V), while others contain both E. coli and also Proteus mirabillis, Proteus vulgaris, Klebsiella pneumoniae, Morganella morganii, Pseudomonas aeruginosa and Enterococcus faecalis (Solco-Urovac®, StroVac®...). These different compositions partly reflect the epidemiology of the origin countries of these vaccines. This is again a pitfall for the generalisation of the vaccine, since, for optimal effectiveness, each country should develop a vaccine that is closest to its own epidemiology.
The latest guidelines from the EAU strongly recommend the use of immunoactive prophylaxis in rUTIs prevention [8]. The French guidelines are in favor, but without formally recommending it insofar, as these vaccines are not marketed in France [7]. The British and American guidelines do not mention it [18], [19].
We selected 7 first reading studies: 3 meta-analyses and 4 clinical trials. 3 clinical trials were deleted from the final analysis (1 non-randomised open-label trial and 2 studies already included in the meta-analyses).
In the meta-analysis of Bauer et al., the goal was to demonstrate the positive impact of the Uro-Vaxom® vaccine (composed of 18 E. coli strains) versus a placebo. Uro-Vaxom® is a vaccine administered orally daily for 3 months. the analysis of 5 randomised trials, comprising a total of 601 women, found a significant reduction in infectious episodes in favor of the vaccine, although the number of UTIs remained higher compared with long-term antibiotic prophylaxis. The tolerance of the vaccine was also excellent despite some digestive disorders and skin reactions. It should be noted, however, that the studies chosen are sometimes heterogeneous and that some missing information could affect the conclusions of this study [26].
In the meta-analysis of Naber et al., the aim was to prove the effectiveness and safety of using immunoactive prophylaxis to prevent rUTIs. In the OM-89 analysis, the mean number of UTIs per year was significantly reduced versus the placebo (0.77 vs 1.27, a difference of 39.4%). However, we can be surprised at the low number of UTIs in the placebo group in untreated women who should have at least 3 episodes per year. Again, the vaccine was well tolerated. The analysis of intravaginal administration of a suppository (Urovac® type) also found results in favor of this type of immunoactive prophylaxis with a significant decrease of UTIs. Nevertheless, the authors specify that these conclusions are to be qualified by the small numbers of the various selected studies with the necessity of phase III studies. The scheme of Hopkins et al., one administration once a week for 3 weeks (booster cycle), then once a month for 3 months, seems better [27]. There was good tolerance for this type of vaccine despite local reactions such as vaginitis, bleeding, or vaginal rash [28].
In the meta-analysis of Beerepoot et al., the aim was to evaluate the effectiveness, tolerance, and safety of non-antibiotic prophylactic measures. Regarding the analysis of vaccines, the conclusions are the same as the previous two since no new trial has been included. The RR for UTI occurrence when using the OM-89 (Uro-Vaxom®) vaccine was evaluated at 0.61; 95% CI (0.48-0.78). For the Urovac® Vaginal Vaccine, the RR was 0.81, 95% CI (0.68-0.96) [29].
Finally, in the phase 1b trial of Huttner et al., the use of ExPEC4V, a tetravalent bioconjugate vaccine (O1A, O2, O6A and O25B) showed a perfect tolerance of this type of vaccine by intramuscular injection. It was only a phase 1 study about tolerance, so the results deserve to be confirmed. However, ExPEC4V seems to have caused a long-lasting immune response (followed by 9 months in this cohort) with an increase in local IgG production and a decrease of rUTIs, although infections related to vaccine serotypes are not less frequent. We can thus assume a cross immunity [30].
In conclusion, the use of immunoactive prophylaxis is recommended (GoR A, LoE 1a) given its effectiveness and excellent tolerance. In view of the better quality studies available and the greater decline in its use, Uro-Vaxom® is recommended as first line. However, Urovac® may also be prescribed as first-line therapy if the patient wishes, due to an administrative method that requires less long-term observance. The ExPEC4V bioconjugate vaccine appears promising but is still in the development phase and these positive results need to be confirmed by a phase II study.
3.3 Bacterial interference
The concept of bacterial interference appeared in 1975 after the discovery in a young female patient who had been colonized by E. coli strain 83972 for 3 years without the occurrence of UTI. This bacteria, in addition to its phenotypic particularities (absence of surface antigens O and K, flagella, capsule, fimbriae type 1, P or F1C), has the ability to rapidly and durably colonise the urothelium, in particular, by its ability to quickly create a biofilm while remaining non-pathogenic. From this came the idea of using this bacteria to limit the colonisation of the urothelium by uropathogenic bacteria [15], [31].
Bacterial interference as such consists in intra-vesical inoculation of a non-pathogenic bacteria capable of lastingly colonising the urothelium and thus protecting the host from uropathogenic bacteria. The competition is done in many ways. The protective bacteria can either produce an inhibitory substance for uropathogenic bacteria or block their binding sites to the urothelium. Competition can also be related to the pH value or nutrients needed for bacterial growth [31].
This therapeutic strategy has been widely studied in-vitro but the recent in-vivo studies have low levels of evidence, and the majority of these studies were carried out on a population of patients with a neurological bladder at high risk of UTI. We have retained, as a working document, the study of Falcou et al. [31], the only publication to date having conducted a systematic review of the main clinical trials conducted over the last 20 years.
This study has retained, in its qualitative analysis, 7 clinical trials (including 3 randomised) using bacterial strains E. coli 83972 and HU2117. Patients had a neurological bladder with a nonphysiological micturition mode (intermittent or indwelling or suprapubic bladder catheterisation). For all studies, the duration of the follow-up was at least 12 months. The success rate of colonisation after endovesical instillation varied after 1 month from 37.7% to 83.3% and persisted from 1.6 months to 12.3 months. All studies found a significant decrease in the number of UTIs in these patients with neurological bladder: from a maximum of 4 UTIs per patient-year to a minimum of 0 UTI per patient-year depending on the studies. In all of these trials, no serious adverse events or UTIs were reported following inoculation. It is, of course, necessary to note the very great heterogeneity of these studies in their criteria of inclusions or exclusions as much as in the definitions used to describe a UTI or for the protocol of inoculation.
Although these results are promising and in favor of this treatment, it is currently impossible to recommend bacterial interference for the prevention of rUTIs in adult women. First of all, because it concerns studies with a low level of evidence and few participants, but also because it has never been studied in this indication. It is therefore necessary to continue this research, but this concept seems all the more interesting since the appearance of recent work on the urinary microbiota.
3.4 Oestrogens
During menopause, the gradual onset of oestrogen deficiency is likely to lead to rUTIs. Tissue atrophy, secondary to this deficiency, may cause a change in the pelvic static with the eventual occurrence of prolapse itself providing lower urinary tract disorders with bladder emptying disorders [29]. The oestrogen receptors are located in the vaginal mucosa but also in the distal part of the urethra in the epithelial mucosa. The mechanisms are not always clear, but it seems that oestrogen has a direct influence on the contractility of the detrusor but also the urinary sphincter and pelvic muscles. Moreover, they also influence the synthesis of collagen [32]. Finally, oestrogen stimulates the proliferation of Lactobcillus spp, the oestrogenic deficiency resulting from menopause therefore leads to a decrease in this protective flora and an increase in vaginal pH eventually promoting bacterial adhesion, including E. coli, to the vaginal mucosa [33].
At present, only the guidelines of the EAU mention this therapy. The EAU recommends the use of vaginal oestrogen replacement only in postmenopausal women and with a low level of recommendation [8].
We selected 3 studies for this chapter, 2 of which were excluded as they are present in the latest study, which is the most recent meta-analysis found on the subject.
The meta-analysis of Beerepoot et al. found no reduction in the risk of occurrence of rUTIs in the case of an oral administration of oestrogen (RR 1.1, 95% CI 0.78-1.56) (I2 = 2.6%). Given the increased risk of serious adverse effects (myocardial infarction, venous thrombosis ...), this treatment is not recommended for this indication.
The use of a vaginal oestrogen replacement does not significantly reduce the occurrence of rUTI (RR 0.42, 95% CI 0.16-1.10). Nevertheless, the two studies selected for this synthesis use very different protocols (cream for one and intra-vaginal ring for the other) and that is why this result is negative given the coefficient of heterogeneity (I2 = 85.3%). The statistical adjustment does not allow the discovery of a significant difference while the two studies found significant results. The number of patients, however, remains low and the methodology too imperfect to be sure of the effectiveness of this treatment. Adverse effects were seen in almost 20% of women but were limited to local non-serious events such as vaginal irritation or discomfort [29].
Given these elements, it appears that vaginal oestrogen replacement could prevent rUTI. Given its good tolerability and ease of use, vaginal oestrogen replacement may be recommended (GoR A; LoE 1b) for the prevention of rUTI but only in postmenopausal women.
3.5 Probiotics
The use of probiotics was proposed in the late 1980s after it was demonstrated that Lactobacillus spp had inhibitory activity on uropathogenic bacteria through its production of hydrogen peroxide (H2O2). The theory of the effectiveness of this type of treatment is that the colonisation of the perineum and the vagina is an essential step in the occurrence of a UTI. Lactobacillus spp, with the production of lactic acid, help maintain the pH of the vagina around 4.5, very unfavorable for a large majority of uropathogens. In addition, the production of H2O2 and proteolytic enzymes by Lactobacillus spp would limit the colonisation of the vagina by preventing the adhesion of uropathogenic bacteria to epithelial cells [15], [34], [35]. The most studied lactobacillus are L. Rhamnosus, L. reuteri, and L. crispatus, sometimes by oral administration, sometimes by administration of intravaginal suppository.
At present, only the guidelines of the EAU mention this therapy. EAU makes no recommendation for or against its use due to a lack of scientific data [8].
We first selected 4 studies including 2 meta-analyses. We excluded both clinical studies because they were already included in both meta-analyses.
The meta-analysis by Grin et al. was conducted using a Cochrane methodology and included a total of 5 studies and 294 participants. In this study, administration of Lactobacillus spp was performed exclusively by intravaginal suppository. The analysis found a RR favoring the use of probiotics for the prevention of rUTIs but not significant: RR 0.85 (95% CI of 0.58-1.25, p = 0.41). Overall heterogeneity was low with a coefficient I2 = 19% [36].
The Cochrane meta-analysis from Schwenger et al. included 6 studies and 352 participants (including adults and children) with a low overall heterogeneity (I2 = 23%). Again the RR was in favour of probiotics for the prevention of rUTIs but this result was not statistically significant: RR 0.82, 95% CI 0.60 to 1.12 [37].
The authors of these two meta-analyses insist that other larger studies are needed to validate this therapy. Indeed, the difficulty concerning this subject is the lack of large-scale studies, as well as the diversity of the Lactobacillus strains used and the route of administration whose effectiveness, at present, cannot be certain. The adverse effects reported for probiotics which administered intravaginally are also rare and of a local reaction type. In addition, the duration of the administration scheme (very heterogeneous according to the studies) is not yet clearly established. Finally, the positive study on reducing rUTIs included women with non-symptomatic bacteriuria and a very heterogeneous population with many inclusion biases.
For all these reasons, and despite its safety, probiotics cannot currently be recommended for the prevention of rUTIs.
3.6 Endovesical instillation of hyaluronic acid
The use of hyaluronic acid (HA) for the prevention of rUTIs has appeared relatively recently even though research on glycosaminoglycans (GAG) began in the 1970s. GAGs provide a protective barrier to the urothelium that protects it, both against the urinary electrolytes but also against the adhesion of bacteria [38], [39], [40], [41]. Based on this assumption, it has been proposed that the occurrence of rUTIs could be favoured by a deficit in GAG that could be restored via the use of different products such as heparin, chondroitin sulphate (CS), pentosan polysulphate, and HA [39]. From this was born the idea of endovesical instillation of these different products, the HA being so far the most studied.
At present, only the guidelines of the EAU mention this therapy. EAU makes no recommendation for or against its use due to a lack of scientific data [8].
We selected 3 studies including 1 meta-analysis and a review of the literature. The 3rd article was excluded since it was analysed in the other two.
In the meta-analysis of De Vita et al., 4 studies were included in the analysis, including two randomised and prospective ones. The use of HA associated with CS found a significant decrease in the number of UTIs per patient per year (MD -2.45, 95% CI -4.63 to -0.28, p = 0.03) but with a very high overall heterogeneity (I2 = 94%) and an analysis covering only 2 of the 4 trials, which limits the scope of this result [42].
In the literature review by Madersbacher et al., which was not a systematic review of the literature, the included studies again contained very heterogeneous studies in both the protocols, the products used, and the study design. The authors' conclusion was that despite a seemingly beneficial effect on rUTIs prevention, the low level of evidence from the included studies did not support a conclusion [43].
In the light of these two studies, therefore, it does not seem possible, at present, to recommend the use of any of these products for the prevention of rUTIs.
3.7 Other measures
Other measures have been studied to reduce the occurrence of rUTIs, however, the effectiveness of these measures is not proven today.
Classically recommended behavioural modifications, such as post-coital urination, increasing fluid intake, wiping from front to back after defecation, wearing cotton underwear and reduced douching, are measures that have been hardly evaluated in the literature, except in the study of Scholes et al. which did not seem to find any benefit from these modifications [44].
Although these measures are safe, some may appear to be restrictive for some patients and there is no reason to recommend them in the current state of knowledge (GoR B, LoE 2a). Nevertheless, a thorough interrogation will make it possible to identify the patients with bad habits whose implication in the genesis of rUTIs appears obvious and to whom these measures can be recommended.
Acupuncture has also been tried in this indication, however, clinical trials are few and the methodology used cannot be certain of its effectiveness and cannot recommend this type of treatment despite positive results [29].
Similarly, the use of D-mannose, whose therapeutic action limits the adhesion of bacteria to the urothelium, which has shown an effectiveness comparable to nitrofurantoin in terms of prevention of rUTI [45], cannot currently be recommended given the lack of confirmation of these promising results.
Finally, the review Cochrane by Flower et al. has not proven conclusively the effectiveness, alone or as an adjunct to antibiotics, of traditional Chinese herbal medicines in herbal teas, pills or granules. Despite effectiveness with a RR 0.28 CI (0.09 to 0.82), the methodological weakness of the 7 included studies does not allow us to conclude and recommend this treatment for rUTI prevention [46].
4 Further Research
The methods proposed to try to reduce rUTIs are numerous and, in general, scientifically well supported. The challenge is twofold: to improve the quality of life of these patients, while limiting the prescription of antibiotics in the long term. Unfortunately, many of these non-antibiotic measures turn out to be alternatives that are often less effective than antibiotics, which remains the reference.
The paradox of this pathology is to propose as many therapeutic alternatives for a pathology without gravity but source of many difficulties for the patients as well as for the practitioners who take care of them.
The realisation of high quality clinical trials seems essential today to provide definitive answers to some measures that have been studied for over 20 years but whose effectiveness remains uncertain.
Finally, the recent work about the urinary microbiota appears to be an essential step in understanding this complex pathology whose physiopathology is still uncertain. In this sense, bacterial interference appears to be a promising treatment even though its clinical applicability is still in its infancy.
5 Conclusions
The prevention of rUTI using non-antimicrobial prophylactic measures seems promising, but is still hampered by the lack of scientific data for some of them. Currently, only cranberry, immunoactive prophylaxis, and vaginal oestrogen replacement in postmenopausal women are measures that can be recommended for the prevention of rUTIs in women. These measures, which can be used alone or combined with antibiotic prophylaxis, may improve the quality of life for these patients.
6 Acknowledgement
Maxime Vallée acknowledges financial support from the European Association of Urology and the French Association of Urology.
7 Conflict of interest of each author
The authors have nothing to disclose.
References
[1] Wagenlehner F, Wullt B, Ballarini S, Zingg D, Naber KG. Social and economic burden of recurrent urinary tract infections and quality of life: a patient web-based study (GESPRIT). Expert Rev Pharmacoecon Outcomes Res. 2018 Feb;18(1):107-17. DOI: 10.1080/14737167.2017.1359543[2] Ennis SS, Guo H, Raman L, Tambyah PA, Chen SL, Tiong HY. Premenopausal women with recurrent urinary tract infections have lower quality of life. Int J Urol. 2018 Jul;25(7):684-9. DOI: 10.1111/iju.13698
[3] Renard J, Ballarini S, Mascarenhas T, Zahran M, Quimper E, Choucair J, et al. Recurrent lower urinary tract infections have a detrimental effect on patient quality of life: a prospective, observational study. Infect Dis Ther. 2014 Dec 18;4(1):125-35. DOI: 10.1007/s40121-014-0054-6
[4] Huttner A, Kowalczyk A, Turjeman A, Babich T, Brossier C, Eliakim-Raz N, et al. Effect of 5-Day Nitrofurantoin vs single-dose Fosfomycin on clinical resolution of uncomplicated lower urinary tract infection in women: a randomized clinical trial. JAMA. 2018 May 1;319(17):1781-9. DOI: 10.1001/jama.2018.3627
[5] Falagas ME, Kotsantis IK, Vouloumanou EK, Rafailidis PI. Antibiotics versus placebo in the treatment of women with uncomplicated cystitis: a meta-analysis of randomized controlled trials. J Infect. 2009 Feb;58(2):91-102. DOI: 10.1016/j.jinf.2008.12.009
[6] Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010 Dec;7(12):653-60. DOI: 10.1038/nrurol.2010.190
[7] Caron F, Galperine T, Flateau C, Azria R, Bonacorsi S, Bruyère F, et al. Practice guidelines for the management of adult community-acquired urinary tract infections. Med Maladies Infect. 2018 Aug [cited 2018 Aug 31];48(5):327-58. DOI: 10.1016/j.medmal.2018.03.005
[8] Bonkat G, Pickard R, Bartoletti R, Bruyère F, Geerlings S, Wagenlehner F, et al. EAU Guidelines on urological infections. 2017 [cited 2017 Oct 13]. Available from: http://uroweb.org/wp-content/uploads/19-Urological-infections_2017_web.pdf
[9] World Health Organization. Antimicrobial resistance: global report on surveillance. Geneva: World Health Organization; 2014. Available from: https://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf
[10] Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018 Apr 10;115(15):E3463-70. DOI: 10.1073/pnas.1717295115
[11] Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract – a role beyond infection. Nat Rev Urol. 2015 Feb;12(2):81-90. DOI: 10.1038/nrurol.2014.361
[12] Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. DOI: 10.1371/journal.pmed.1000097
[13] Guay DRP. Cranberry and urinary tract infections: drugs. 2009;69(7):775-807. DOI: 10.2165/00003495-200969070-00002
[14] Gupta A, Dwivedi M, Mahdi AA, Nagana Gowda GA, Khetrapal CL, Bhandari M. Inhibition of adherence of multi-drug resistant E. coli by proanthocyanidin. Urol Res. 2012 Apr;40(2):143-50. DOI: 10.1007/s00240-011-0398-2
[15] Bruyère F, Boiteux J-P, Sotto A, Karsenty G, Bastide C, Guy L, et al. Les traitements anti-infectieux non médicamenteux en urologie [Anti-infective treatments in urology]. Prog Urol. 2013 Nov;23(15):1357-64. DOI: 10.1016/j.purol.2013.09.002
[16] Epp A, Larochelle A. Recurrent urinary tract infection. J Obstet Gynaecol Can. 2010 Nov [cited 2018 Jul 14];32(11):1082-90. DOI: 10.1016/S1701-2163(16)34717-X
[17] Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011 Oct;5(5):316-22. DOI: 10.5489/cuaj.687
[18] Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011 Mar 1;52(5):e103-20. DOI: 10.1093/cid/ciq257
[19] National Institute for Health and Care Excellence. Urinary tract infections in adults. 2015 Jun 11 [cited 2018 Jul 9]. Available from: https://www.nice.org.uk/guidance/qs90/resources/urinary-tract-infections-in-adults-pdf-2098962322117
[20] Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012 Oct [cited 2018 Jul 14];10(10):CD001321. DOI: 10.1002/14651858.CD001321.pub5
[21] Jepson RG, Mihaljevic L, Craig JC. Cranberries for treating urinary tract infections. Cochrane Database Syst Rev. 1998 [cited 2018 Jul 27];1998(2):CD001322. DOI: 10.1002/14651858.CD001322
[22] Jepson RG, Mihaljevic L, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2004 Apr 19;(2):CD001321. DOI: 10.1002/14651858.CD001321.pub3
[23] Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD001321. DOI: 10.1002/14651858.CD001321.pub4
[24] Ranfaing J, Dunyach-Remy C, Louis L, Lavigne JP, Sotto A. Propolis potentiates the effect of cranberry (Vaccinium macrocarpon) against the virulence of uropathogenic Escherichia coli. Sci Rep. 2018 Jul [cited 2018 Jul 24];8(1):10706. DOI: 10.1038/s41598-018-29082-6
[25] Winer JH. Resistant urinary tract infections – combined autogenous vaccine and drug therapy. Calif Med. 1956 Mar;84(3):204-5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1532924/pdf/califmed00267-0124.pdf
[26] Bauer HW, Rahlfs VW, Lauener PA, Blessmann GSS. Prevention of recurrent urinary tract infections with immuno-active E. coli fractions: a meta-analysis of five placebo-controlled double-blind studies. Int J Antimicrob Agents. 2002 Jun;19(6):451-6. DOI: 10.1016/S0924-8579(02)00106-1
[27] Hopkins WJ, Elkahwaji J, Beierle LM, Leverson GE, Uehling DT. Vaginal mucosal vaccine for recurrent urinary tract infections in women: Results of a phase 2 clinical trial. J Urol. 2007 Apr;177(4):1349-53. DOI: 10.1016/j.juro.2006.11.093
[28] Naber KG, Cho YH, Matsumoto T, Schaeffer AJ. Immunoactive prophylaxis of recurrent urinary tract infections: a meta-analysis. Int J Antimicrob Agents. 2009 Feb;33(2):111-9. DOI: 10.1016/j.ijantimicag.2008.08.011
[29] Beerepoot MA, Geerlings SE, van Haarst EP, van Charante NM, ter Riet G. Nonantibiotic prophylaxis for recurrent urinary tract infections: a systematic review and meta-analysis of randomized controlled trials. J Urol. 2013 Dec;190(6):1981-9. DOI: 10.1016/j.juro.2013.04.142
[30] Huttner A, Hatz C, van den Dobbelsteen G, Abbanat D, Hornacek A, Frölich R, et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: a randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect Dis. 2017 May;17(5):528-37. DOI: 10.1016/S1473-3099(17)30108-1
[31] Falcou L, Davido B, Even A, Bouchand F, Salomon J, Sotto A, et al. Stratégie préventive originale des infections urinaires symptomatiques chez les patients porteurs d’une vessie neurologique : l’interférence bactérienne, état de l’art [Original strategy for prevention of recurrent symptomatic urinary tract infections in patients with neurogenic bladder: bacterial interference, state of the art]. Prog Urol. 2018 May;28(6):307-14. DOI: 10.1016/j.purol.2018.03.002
[32] Robinson D, Cardozo LD. The role of oestrogens in female lower urinary tract dysfunction. Urology. 2003 Oct;62(4):45-51. DOI: 10.1016/s0090-4295(03)00676-9
[33] Raz R. Postmenopausal women with recurrent UTI. Int J Antimicrob Agents. 2001 Apr;17(4):269-71. DOI: 10.1016/S0924-8579(00)00355-1
[34] Czaja CA, Stapleton AE, Yarova-Yarovaya Y, Stamm WE. Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infect Dis Obstet Gynecol. 2007 [cited 2018 Jul 31];2007:35387. DOI: 10.1155/2007/35387
[35] Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011 May;52(10):1212-7. DOI: 10.1093/cid/cir183
[36] Grin PM, Kowalewska PM, Alhazzan W, Fox-Robichaud AE. Lactobacillus for preventing recurrent urinary tract infections in women: meta-analysis. Can J Urol. 2013 Feb;20(1):6607-14. Available from: https://pubmed.ncbi.nlm.nih.gov/23433130/
[37] Schwenger EM, Tejani AM, Loewen PS. Probiotics for preventing urinary tract infections in adults and children. Cochrane Database Syst Rev. 2015 Dec [cited 2018 Jul 6];(12). DOI: 10.1002/14651858.CD008772.pub2
[38] Damiano R, Quarto G, Bava I, Ucciero G, De Domenico R, Palumbo MI, et al. Prevention of recurrent urinary tract infections by intravesical administration of hyaluronic acid and chondroitin sulphate: a placebo-controlled randomised trial. Eur Urol. 2011 Apr;59(4):645-51. DOI: 10.1016/j.eururo.2010.12.039
[39] Lipovac M, Kurz C, Reithmayr F, Verhoeven HC, Huber JC, Imhof M. Prevention of recurrent bacterial urinary tract infections by intravesical instillation of hyaluronic acid. Int J Gynecol Obstet. 2007 Mar;96(3):192-5. DOI: 10.1016/j.ijgo.2006.11.025
[40] Constantinides C, Manousakas T, Nikolopoulos P, Stanitsas A, Haritopoulos K, Giannopoulos A. Prevention of recurrent bacterial cystitis by intravesical administration of hyaluronic acid: a pilot study. BJU Int. 2004 Jun;93(9):1262-6. DOI: 10.1111/j.1464-410X.2004.04850.x
[41] Cicione A, Cantiello F, Ucciero G, Salonia A, Torella M, De Sio M, et al. Intravesical treatment with highly-concentrated hyaluronic acid and chondroitin sulphate in patients with recurrent urinary tract infections: results from a multicentre survey. Can Urol Assoc J. 2014;8(9-10):E721-7. DOI: 10.5489/cuaj.1989
[42] De Vita D, Antell H, Giordano S. Effectiveness of intravesical hyaluronic acid with or without chondroitin sulfate for recurrent bacterial cystitis in adult women: a meta-analysis. Int Urogynecol J. 2013 Apr;24(4):545-52. DOI: 10.1007/s00192-012-1957-y
[43] Madersbacher H, van Ophoven A, van Kerrebroeck PE. GAG layer replenishment therapy for chronic forms of cystitis with intravesical glycosaminoglycans – a review. Neurourol Urodyn. 2013 Jan;32(1):9-18. DOI: 10.1002/nau.22256
[44] Scholes D, Hooton TM, Roberts PL, Stapleton AE, Gupta K, Stamm WE. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000 Oct;182(4):1177-82. DOI: 10.1086/315827
[45] Kranjčec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014 Feb;32(1):79-84. DOI: 10.1007/s00345-013-1091-6
[46] Flower A, Wang LQ, Lewith G, Liu JP, Li Q. Chinese herbal medicine for treating recurrent urinary tract infections in women. Cochrane Database Syst Rev. 2015 Jun [cited 2017 Sep 13];2015(6):CD010446. DOI: 10.1002/14651858.CD010446.pub2




