Prevention of prematurity by early diagnosis and treatment of bacterial urogenital infections – The role of bacterial vaginosis
1 Department of Gynecology and Obstetrics, HELIOS Hospital Erfurt Ltd., Erfurt, Germany
Abstract
Bacterial vaginosis has a significant relative risk for miscarriage or prematurity of 1.4–6.9%. In the initial Erfurt trial 0.3% of the neonates with gestational age <32 + 0 weeks were seen in an intervention group vs. 3.3% (p <.01) in the control group; in the Thuringia campaign the figures were 0.94% vs. 1.36% (p <.01) for the entire state. The rate of newborns <1,000 g was reduced to 0.38%, the lowest incidence ever seen in any of the German states in the past.
The
Now, following correction, detailed analysis and extensive discussion of the data a model trial of four health insurance providers in five German states was initiated, aimed at scientific evaluation as well as cost-benefit analysis. The preliminary new data and calculations also signal substantial savings as well as implementation of new simplified pH-measurement techniques and tools.
Prevention of prematurity by screening, diagnosis and therapy of genital infections is without any doubt a step for optimising and rationalising the health care system. Also the noncommensurable extraordinary strain on all parties involved could be reduced in a most beneficial way. Similar developments take place abroad: the described innovative pH measurement regime is introduced in more than 20 countries so far.
Note
This is an updated manuscript as published earlier in:
Naber KG, Schaeffer AJ, Heyns CF, Matsumoto T, Shoskes DA, Bjerklund Johansen TE, eds. International Consultation on Urogenital Infections. Arnhem: European Association of Urology - International Consultation on Urological Diseases; 2010. Available from: http://www.icud.info/urogenitalinfections.html
Summary of recommendations
- Routine screening for N. gonorrhoeae, C. trachomatis and bacterial vaginosis is indicated in defined populations because of infection associated miscarriage and preterm birth as well as postpartum complications (GoR B).
- The intravaginal pH self-measurement as a screening procedure followed by antimicrobial therapy in case of bacterial vaginosis is a significant step in reduction of prematurity (GoR B).
1 Introduction
Ectopic pregnancy, spontaneous abortion and stillbirth, prematurity, congenital and perinatal infections as well as puerperal maternal infections represent outcomes of pregnancy in which bacterial (sexually transmitted) infectious agents play an important etiologic role, especially in pregnant adolescents and young adults compared to older pregnant women, who are more likely to be involved in a stable monogamous relationship [1]. The prevalence of bacterial sexually transmitted infections (STI), such as with Chlamydia trachomatis and Neisseria gonorrhoeae is in general highest among women younger than 25 years; Mycoplasma genitalium, leptotrichia, and other still uncultivable organisms may prove to be important in the future.
The anatomy and physiology of the urogenital tract change dramatically during pregnancy: The glycogen content of the we urethral and vaginal epithelium increases, the intravaginal pH decreases, the columnar epithelium of the cervix hypertrophies and is exposed to the changed local microbial flora and/or infection. This leads to an increasing risk of urinary tract infections as well as chorioamnionitis and amniotic fluid infection in the course of pregnancy and following delivery [2], [3], [4].
Since C. trachomatis, N. gonorrhoeae, and bacterial vaginosis (BV) have been associated with an increased risk of febrile morbidity after surgical abortion routine screening is indicated prior to the procedure in most populations [5], [6], but also considered in general for pregnancy [1], [7], [8]. Pregnancy loss and preterm birth comprise the spectrum of adverse pregnancy outcomes following urogenital infections Especially BV has increasingly been recognized as cause of prematurity all over the world, e.g. in Germany, Austria, Brazil, Greece, Sweden, Denmark, the US, Iran, Korea [8], [9], [10], [11], [12], [13], [14], [15], [16]. Integrating a simple infection screening programme into routine antenatal care can lead to a significant reduction in preterm births and reduces the rate of late miscarriage. In an intervention group (n=2,058) the number of preterm births was significantly lower than in controls (n=2,097), (3.0% vs. 5.3%; 95% CI 1.2–3.6; p=0.0001). The number of late miscarriages was 8 vs. 15 [9].
2 Methods
A systematic literature search was performed in Pub Med beginning with 2004 with the following key words: prevention of prematurity, intravaginal pH-measurement, genital infections, and the following limitations: English abstract available, non-systemic infection.
A total of 110 publications was identified, which were screened by title and abstract. After exclusion of duplicates a total of 8 were included into the analysis, supplemented by 31 citations known to the author. It has to be considered, however, that prevention of prematurity caused by infection is a fairly new emerging task. Therefore the literature is still very limited.
The studies were rated according to their level of evidence (LoE) and the grade of recommendation (GoR) using ICUD standards (for details see Preface) [17], [18].
3 The current challenge
Children born prematurely in general, and especially children with low birthweight, run a high risk of health and development problems. Despite this fact and despite all interventional efforts prematurity rates have remained unchanged for years e.g. at about 6–8% throughout Germany. In contrast, both perinatal morbidity and mortality have been reduced significantly over the last years. With the current high standard of neonatal medicine a further improvement of those results can only be expected if we succeed in reducing prematurity as the main contributor to perinatal morbidity and mortality [19]. Because of the preventive character of all measures this is not only a medical, but also a social-political task!
4 Definition of the disease
Textbooks for obstetrics name a number of factors as causes for prematurity. The type in question here is (the more and more preventable) infection-based premature births. Currently the quantitatively relevant, adjustable and scientifically proven causes of prematurity are general infections of the vagina and cervix uteri, in particular in the latter those caused by Chlamydia trachomatis (LoE 2a). As a logical consequence e.g. since April 1, 1995 screening for Chlamydia in pregnancy is obligatory in Germany [7] (GoR B).
Intriguing data implicating bacterial infectious agents in prematurity were first derived from a study performed more than 40 years ago [20] investigating the role of urinary tract infection in prematurity in 279 nonbacteriuric women allocated to treatment with six weeks of oral tetracycline at 1 g per day (today considered contraindicated in pregnancy) or with placebo prior to 32 weeks of gestation (Table 1). The results showed that the tetracycline–treated group had significantly longer gestations, fewer preterm infants, fewer episodes of postpartum fever, and fewer neonatal complications in comparison with the placebo-treated group (LoE 1b). Despite all limitations of the study this benefit suggested the presence of unidentified microorganisms in the urogenital tract (C. trachomatis or BV-associated) that could have contributed to prematurity in the placebo group. This raises the possibility that also other as-yet unidentified organisms may be associated with infection and prematurity, since many bacteria produce phospholipases that may directly damage the membranes or lead to prostaglandin production [1], [21]. This process cannot be reversed at late stage by antibiotics, so the best strategy is prevention of these conditions by early detection and treatment of lower urogenital or genital infection (GoR B).
|
Measurement |
Tetracycline |
Placebo |
p |
|---|---|---|---|
|
Mean gestation (weeks) |
39.1 |
38.1 |
<0.025 |
|
Mean birth weight (g) |
3,277 |
3,141 |
NS* |
|
|
% |
% |
|
|
Premature liveborn |
5.4 |
15.2 |
<0.025 |
|
Stillborn |
1.4 |
0.8 |
|
|
PROM |
0 |
13 |
NS* |
|
Postpartum fever |
6 |
12 |
<0.001 |
|
Neonatal resuscitation |
8 |
19 |
<0.005 |
|
Respiratory distress |
|
7 |
<0.05 |
|
*Nonsignificant |
|||
Pregnant women should be screened for urinary tract infection during pregnancy as well as for C. trachomatis, possibly N. gonorrhoeae, syphilis and also facultatively T. vaginalis on their first prenatal visit and treated if positive (GoR B). Screening should be repeated in high risk groups [1], [7], however, most recommendations for management and therapy in pregnancy lack support by scientifically performed prospectively randomized studies due to methodical and ethical reasons.
5 Clinical evaluation: Starting point of a broad scale prematurity preventional regime
Bacterial vaginosis, an anaerobic dysbiosis, has a significant prevalence in pregnancy of up to 20% and a relative risk for miscarriage or prematurity of 1.4–6.9% (LoE 2a). An adequate treatment regime, especially in the early stages of pregnancy, can lead to evident reduction of prematurity (GoR B) [8], [22], [23], [24]. The unquestionable efficacy of the concept which the pregnant women actively take part in has been investigated in the prospective Erfurt and Thuringia trials [8]: The objective of these trials was to prove the efficacy of vaginal pH screening during pregnancy, carried out twice per week by the woman herself, because gynaecologistsʼ examinations in the alleged intervals (e.g. every 4 weeks during early pregnancy) are not considered to be sufficient. It was not intended to investigate the well-studied impact of bacterial vaginosis on prematurity, to collect new epidemiological data, to reassess diagnostic measures or to compare different treatment regimes.
Briefly, the patients were recruited into the program with the help of a simple explanatory leaflet at the end of the 12th week of pregnancy. Patients received examination gloves (CarePlan ® VpH, Selfcare, then Inverness GmbH, Munich, now Unipath Diagnostics GmbH, Cologne) and a simple documentation sheet. Each patient was asked to consult her gynaecologist immediately when risk indicators or symptoms relevant for prematurity occured, especially when she found an elevated pH value >4.7. The sensitivity of a pH >4.7 with respect to bacterial vaginosis is 97%; a pH value >4.7 is only 67% specific and not sensitive for other infections [25].
During any unscheduled consultation due to above mentioned indications and after pH control, odour test and wet mount microscopy, the gynaecologist had to decide if the elevated pH was attributable to:
- a physiological condition,
- pH elevation without evident affection or pathogen present,
- bacterial vaginosis or
- a result indicating hospital assignment, such as rupture of the membranes.
Accordingly, following the gynaecologic assessment, the patient was assigned to:
- no therapy,
- a therapy recommendation for a preparation containing lactobacilli (in the Erfurt and Thuringia trial Gynoflor ®, Nourypharma, Oberschleißheim) i. vag.,
- a treatment recommendation for clindamycin cream (SobelinCreme ®, Pharmacia & Upjohn, Erlangen) i. vag., or
- hospital assignment.
6 Risk assessment and reduction
381 women participated in this pilot screening and treatment programme [8]; 2,341 women did not participate, and served as a control group. An early prematurity rate of 0.3% amongst participants was observed, compared to a 2.2% rate amongst women who did not participate, but whose physicians were informed of and taking part in the programme and a 4.1% rate in a group of pregnant women who recieved only conventional prenatal care (Table 2). Compliance with the test glove and with the testing regime was rated as outstanding by all parties involved. Many women emphasized the value of the individuality of the self precaution and as result the change from being object of care during pregnancy to subject.
|
Delivery |
Participants |
Controls A |
Controls B |
|---|---|---|---|
| (n=381) | (n=1,001) | (n=1,340) | |
|
>37 + 0 |
91.9% |
90.7% |
85.4% |
|
32 + 0/36 + 6 |
7.9% |
7.1% |
10.4% |
|
<32 + 0 |
0.3% |
2.2% |
4.1% |
Another 6 months trial was conducted in the federal state of Thuringia starting March 1, 2000. The objective of the trial was to involve all pregnant women throughout the federal state (about 16,000 per year) for a defined period of time with the help of office based gynaecologists and to achieve a measureable decrease in prematurity rates during the annual perinatal state inquiry which had previously been reported stable between 1992 and 1999 [8].
The method was to send office based gynaecologists test glove kits (total 3,833) free of charge. Women who were at least 12 + 0 weeks pregnant were recruited for the campaign. Due to the presumed efficacy of the action it was hypothetically expected that there would be a significant decrease in the prematurity rate. Treatment with lactobacilli or antibiotics was suggested, but was at the discretion of the treating physician. Acidification of the vagina with acid- containing pharmaceutical preparations was, excluded, however, because of the lack of scientific evidence supporting this treatment.
The evaluation of the perinatal inquiry for the year 2000 in Thuringia (16,276 births) gave a clear result: the total number of early premature births was less in the 2nd half of the year according to the initially formulated hypothesis (Figure 1a). Similar results were obtained when comparing the birth weights (Figure 1b). A detailed analysis of participants reporting back (n=607) vs. controls (1. half year 2000, n=7,870) (Hübner, J., VII. Meeting European Society for Infectious Diseases in OB/GYN (ESIDOG), 13.06.2003, Erfurt ) revealed a reduction in:
- prematurity <32 + 0 wks. (0.3% vs. 1.58%; p <.05)
- prematurity <37 + 0 wks. (5.3% vs. 8.5%; p <.01)
- the incidence of newborns <2,500 g (3.45% vs. 6.92%; p <.001).
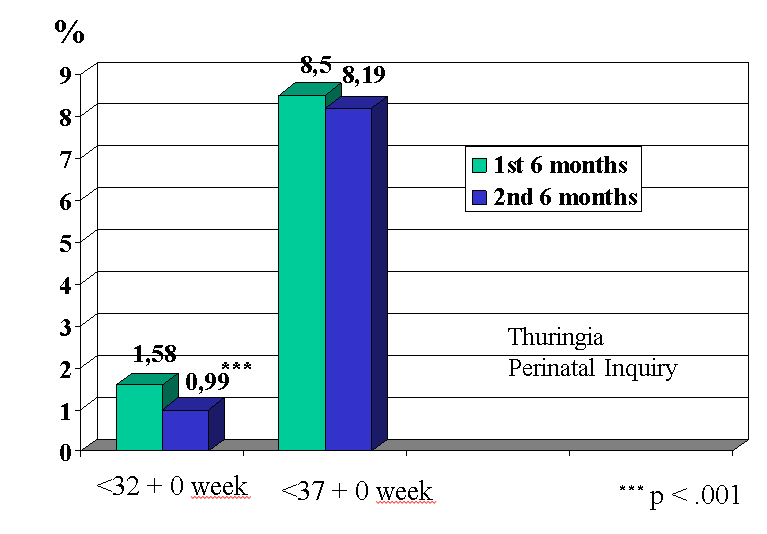
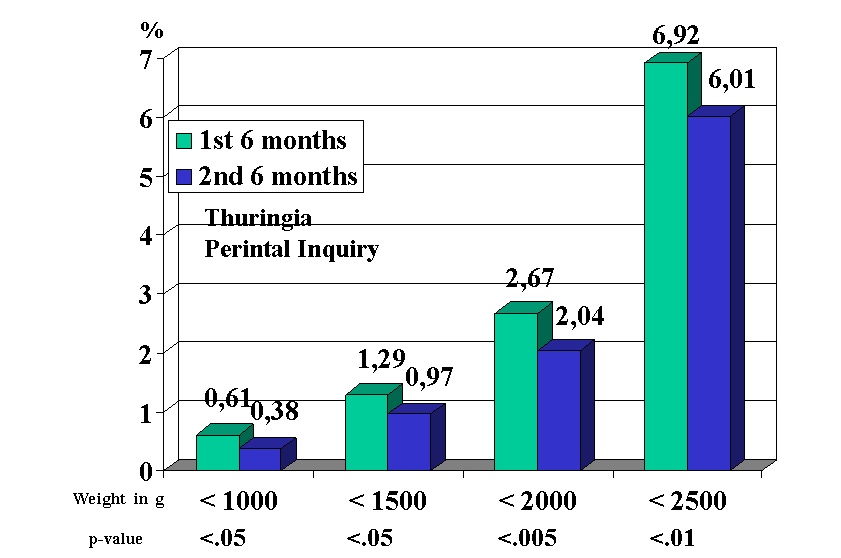
These data allow the conclusion that with this strategy, even if only <50% of the pregnant women participate in a wider territory, the number of early premature births can be significantly reduced. One possible explanation for the success of this programme was that whereas more than one third of early premature births were connected with early rupture of membranes in the first half of the year, this was only the case in one in five during the second half (Figure 2).
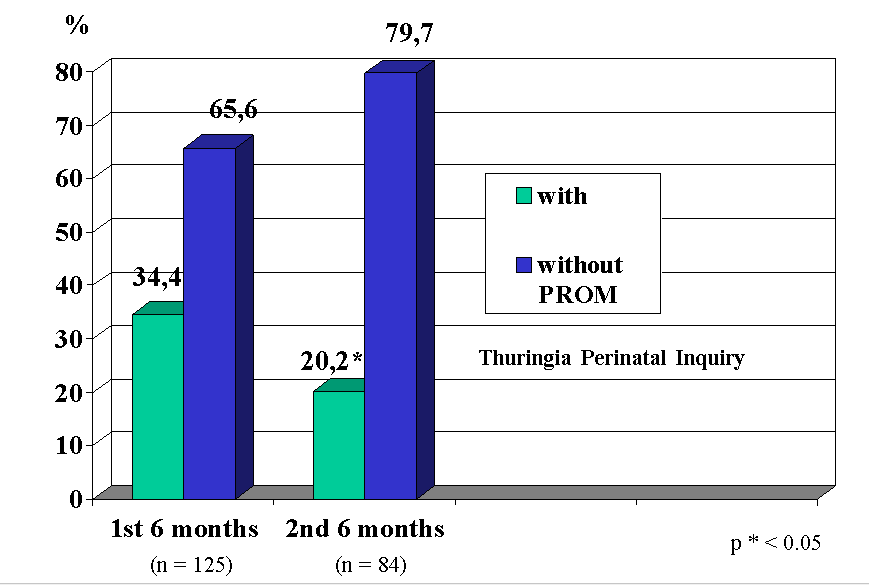
7 Discussion of treatment
The list of risk factors for prematurity is lengthy. The majority of these factors is difficult to influence. Cervical and vaginal conditions are factors which have been scientifically studied to a great extent with regard to their quantitative and qualitative consequences, amongst which the release of bacterial proteases, the release of phospholiphase A2 from membranes, lysosomes and bacteria (Bacteroides and Prevotella spp.), and the resulting synthesis of prostaglandines can be relevant (LoE 2a). Another factor is the direct effect of bacterial endotoxin as well as macrophage activation with release of cytokines [24], [26]. Furthermore, there are indications that fetal cytokines alone can trigger birth; in this situation without maternal reaction to infection, labour can be induced, which is lifesaving for both mother and child can be induced (LoE 2b) [21].
Bacterial vaginosis as dysbiosis rsp. dysbalance with an increase in vaginal anaerobic bacteria concentrations by 1,000-fold is without doubt a risk for prematurity. The large American VIP trial (in which enrolment was later in the course of pregnancy) found a relative rate of 1.6 and therefore as high as for Chlamydia infections or similar to trichomoniasis at 1.7 (LoE 2a) [22]. Aerobic types of colpitis with an increase of the pH value [27] as well as intra- and extraamnial infections with enteropharyngeal pathogens are also thought to contribute towards prematurity (LoE 2b) [28], [29].
In the majority of trials concerning pregnancy, bacterial vaginosis has been successfully treated with Clindamycin rsp. or alternatively with Metronidazole [30], [31], [32]. Even when labour was already established a significant prolongation of pregnancy could be demonstrated in controlled trials (LoE 1b) [26]. Dennemark et al. [33] were able to show that by early intervention either with lactobacillus preparations or preferably with intravaginal Clindamycin treatment a distinct reduction of prematurity could be obtained (LoE 2a). A systemic review and metaanalysis of five randomised controlled trials that comprised 2,346 women led to the conclusion that clindamycin administered at <22 weeks of gestation was associated with a significantly reduced risk of preterm birth at <37 weeks of gestation and late miscarriage in women with abnormal vaginal flora [34]. In principle lactobacillus preparations cannot yet be considered a scientifically proven rsp. causal therapy, but the main objective of treatment in both the Dennemark and the Erfurt/Thuringia investigations was the prolongation of pregnancy.
8 Principles of management
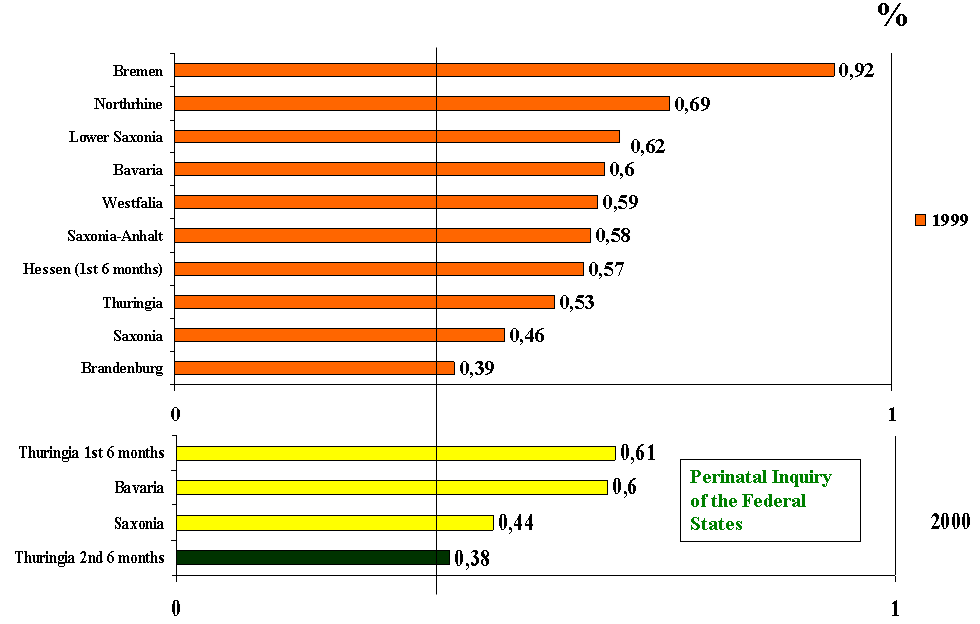
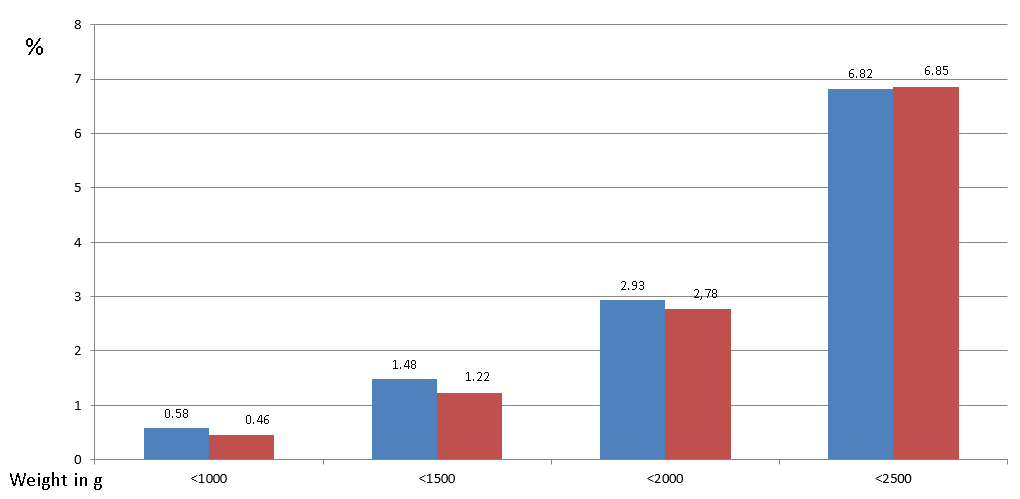
The Erfurt and Thuringia programmes represent for the first time a prospective, controlled study. The main benefit is that because of the active involvement of the pregnant women pH changes could be recognised as early as possible and as a consequence a huge part of the disorders relevant for late miscarriage or premature birth could be addressed with an immediate adaequate therapy. The results confirm the positive consequences of the applied measures in a statistically sound way. As the investigation was carried out in a whole federal state, the positive results can be regarded as a definitive break-through with respect to availability of a practicable and wide spread prevention of prematurity. The comparison with other federal states, i.e. Bavaria or Saxonia, shows that when evaluating the quality indicator birth weight, that the higher rates of prematurity for Thuringia in 1999 could be changed to an unmistakable reduction in the 2nd half of 2000 (Figure 3). The results achieved in Thuringia in 2000 are the best ever seen in any of the German states in the past. However, following discontinuation of the campaign the prematurity rates in Thuringia (monitored till 2005) returned to rates are as high as prior to our state-wide programme in general, as for our hospital in particular (Table 3).
|
Year |
1999 |
I/2000 |
II/2000 |
2001 |
2002 |
2003 |
2004 |
2005 |
|---|---|---|---|---|---|---|---|---|
|
N |
16,233 |
8,162 |
8,458 |
16,408 |
15,995 |
15,436 |
16,058 |
15,633 |
|
<1,000 g |
0.54 |
0.61* |
0.38 |
0.46 |
0.63*** |
0.62** |
0.60** |
0.56** |
|
<1,500 g |
1.22** |
1.29* |
0.97 |
1.09 |
1.32** |
1.17* |
1.15* |
1.30** |
|
<2,000 g |
2.67*** |
2.67* |
2.03 |
2.36** |
2.62*** |
2.74*** |
2.34** |
2.60*** |
|
<2,500 g |
6.76*** |
6.91** |
5.99 |
6.64*** |
6.72*** |
6.80*** |
6.35** |
6.88*** |
|
<1,000 g |
Perinatalcenter |
1.5 |
1.0 |
1.5 |
1.6 |
1.5 |
1.4 |
1.7 |
|
* p<0.05 |
||||||||
Prevention of prematurity by screening, diagnosis und therapy of genital infections is without any doubt an important step for optimising and rationalising the health care system (GoR B). The concepts and results presented are encouraging that an improvement of the situation with simultaneous cost reduction is indeed possible.
9 Further research
The efficacy of the regime is supported and established in several scientifically performed prospective studies.
The data of 7,731 participating pregnant women as well as 135,394 controls are under evaluation right now [27]. In this study, initiated by four German insurance companies, reduction of prematurity will be investigated as well as possible financial savings: Preliminary data indicate about 300 Euros per any pregnancy regardless of outcome, so the possible implementation of the procedure in a guideline is under discussion by the German health authorities.
As further scientific proof for the regime of pH-measurement by the pregnant women themselves as well as the worldwide applicability a prospectively randomized study was initiated in France in 2015 [35].
10 Conclusions for the practice
The investigated concept of pH self measurement leads to an efficient reduction of prematurity and is at the same time a simple means for optimising and rationalising diagnosis as well as therapy. The chance to reduce the extremly costly complications associated with prematurity with minimal expense comes with the fact that the unmeasurable extraordinary strain on all parties involved can be reduced in a beneficial way. Can we as physicians, health care providers and politicians take the liberty to ignore these encouraging prospects?
11 Addendum
Aspects of the Thuringia situation 2017
The Government of the State of Thuringia had decided in 2016 to reestablish a pH selfcare screening program similar to that of the year 2000. According to a sentinel survey at the Department of Ob/Gyn in the central Thuringian town of Arnstadt, beginning at zero in early 2017, almost one year after its initiation about 85% of pregnant women in the state have their vaginal pH measured, however, in contrast to the intention and the successful campaign in 2000 the majority by their obstetricians (Figure 4).
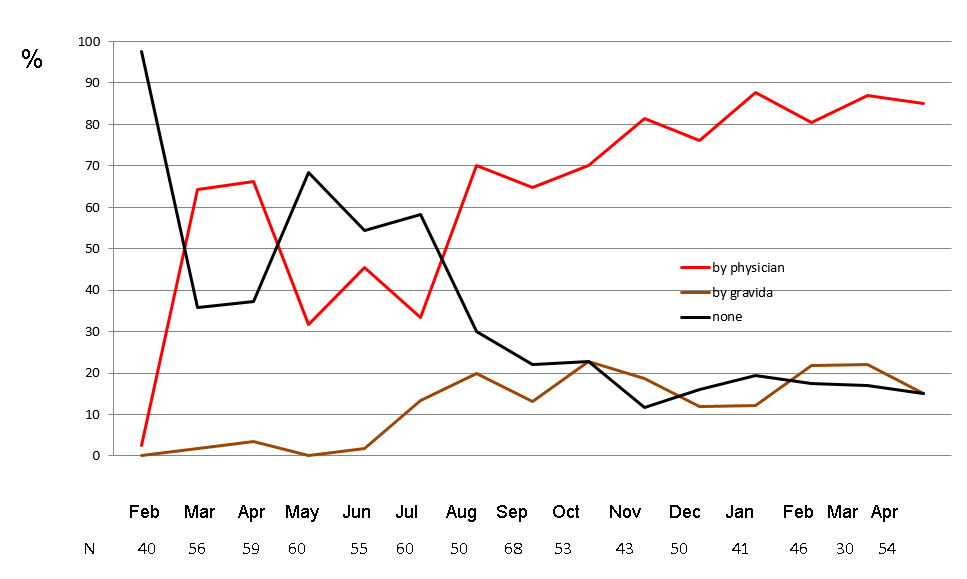
Early preterm birth <32 + 0 wks. in the second half of the year 2017 was obviously reduced as in the previous 2000 results (1.31 vs. 1.46% in controls, n=17,387). The same tendency was seen on the basis of birthweights (Figure 5). This represents a substantial improvement in quality of prenatal care, but is quantitatively falling short of the former 2,000 results, probably due to the low rate of measurement by the women themselves that appears to be more effective by a factor of about 7 according to previous studies. In other words, in the future obstetricians should focus on the integration of pregnant women in order to motivate them to perform self-measurement at least twice every week instead to be measured once a month!
Pro & contra discussion of the recent situation
The well established and investigated screening regime should be implicated in prenatal care as a step of optimizing and rationalizing the national health care system. However, in two decades of discussion it became obvious that the best way to inhibit progress can be to cope with problems by preferring the most complicated medical policies under persistent renunciation of simple solutions. Would a similar caution ever had allowed for instance handwashing according to Semmelweis? That being a 170 years old procedure and never been tested in a scientifically controlled clinical study is free of doubt considered as evidence-based medicine! Nowadays the term Semmelweis effect is a metaphor for the reflex-like tendency to reject new evidence or new knowledge because it contradicts established norms, beliefs or paradigms (Robert Anton Wilson, 1932–2007).
According to the recent literature, we have the potential means for successfully treating women with threatening preterm birth in about 5% of cases. What has to be done? As long as we do not have alternative safe, simple and cheap methods, intravaginal pH-measurement is the best option to detect women at risk and in need for specific diagnostic assessment followed by efficient medical treatment, e.g. by lactobacilli or in case of BV preferably by clindamycin before week 23. The same message was given in other words at the 13th World Congress of Perinatal Medicine in Belgrade in October 2017. Scientific medicine is of no good and l̕art pour l̕art as long as there is no solid fundament. Care for neonates is actually still hampered by deficits in specific medical management, however, prevention of preterm birth is primarily a question of adequate information of all pregnant women and that for also a political one!
So far it remains a vague hope and misunderstanding that in recent practical care worldwide prevention of preterm birth can be substantially improved exclusively on the basis of further scientific studies and discussion. Pregnant women as are neither objects and nor controls, as individuals they deserve rather information and organized counseling than statistical aspects. Obstetrical knowledge and advice has to be biased with political decisions and support, no matter by what party, in what country. Otherwise the current dilemma of preterm birth will remain, with limited benefit and progress in prevention at extreme costs, especially for not sufficiently informed women or in the Third World.
The political campaign in Thuringia was intended to provide specific information to pregnant women and physicians but by no means to interfere with medical competence. Flyers and posters were designed under this aspect repeating messages given by medical guidelines, insurance companies and similar previous programs.
K.I.S.S. (Keep it simple, stupid) is a well known recipe for success. As the lay(wo)man being a patient is able to monitor blood pressure or glucose himself an informed pregnant woman is competent to perform and interpret pH-measurement i.vag. as self-screening. This effective and uncomplicated regime should have the potential to be used by everyone everywhere. The 4th millennium goal missed in 2015 could come closer. On the other hand, any screening alternative superior to the current as challenging replacement is desirable and more than welcome. It had to be judged by sensitivity, specificity, compliance, costs … and “Primum non nocere, secundum cavere, tertium sanare” (Scribonus largus, about 50).
The Thuringia prematurity prevention campaign will be continued in 2018 with special focus on pH-selfmeasurement. Next results should be available by mid of 2019.
12 Conclusions
- By simple means early prematurity can be possibly cut by about half.
- Pregnant women in prenatal care have to be considered also as subject.
- Efficient prevention of preterm birth is a medical as well as a political task.
13 Conflict of Interest
One of the pH-devices used was developed and marketed by the author causing a potential conflict of interest.
References
[1] Hitti J, Watts DH. Bacterial Sexually Transmitted Infections in Pregnancy. In: Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, Cohen MS, Watts DH, editors. Sexually transmitted Diseases. 4th ed. New York: Mc Graw Hill; 2008. p. 1529-61.[2] Singer A. The uterine cervix from adolescence to the menopause. Br J Obstet Gynaecol. 1975 Feb;82(2):81-99. DOI: 10.1111/j.1471-0528.1975.tb02204.x
[3] Arya OP, Mallinson H, Goddard AD. Epidemiological and clinical correlates of chlamydial infection of the cervix. Br J Vener Dis. 1981 Apr;57(2):118-24. DOI: 10.1136/sti.57.2.118
[4] Goplerud CP, Ohm MJ, Galask RP. Aerobic and anaerobic flora of the cervix during pregnancy and the puerperium. Am J Obstet Gynecol. 1976 Dec 1;126(7):858-68. DOI: 10.1016/0002-9378(76)90674-8
[5] Sørensen JL, Thranov I, Hoff G, Dirach J. Early- and late-onset pelvic inflammatory disease among women with cervical Chlamydia trachomatis infection at the time of induced abortion--a follow-up study. Infection. 1994 Jul-Aug;22(4):242-6. DOI: 10.1007/BF01739907
[6] Larsson PG, Platz-Christensen JJ, Thejls H, Forsum U, Påhlson C. Incidence of pelvic inflammatory disease after first-trimester legal abortion in women with bacterial vaginosis after treatment with metronidazole: a double-blind, randomized study. Am J Obstet Gynecol. 1992 Jan;166(1 Pt 1):100-3. DOI: 10.1016/0002-9378(92)91838-2
[7] Hoyme UB. Mutterschaftsrichtlinien und Chlamydienscreening [Prenatal guidelines and Chlamydia screening]. Z Geburtshilfe Neonatol. 1997 Jul-Aug;201(4):113-4.
[8] Hoyme UB, Saling E. Efficient prematurity prevention is possible by pH-self measurement and immediate therapy of threatening ascending infection. Eur J Obstet Gynecol Reprod Biol. 2004 Aug;115(2):148-53. DOI: 10.1016/j.ejogrb.2004.02.038
[9] Kiss H, Petricevic L, Husslein P. Prospective randomised controlled trial of an infection screening programme to reduce the rate of preterm delivery. BMJ. 2004 Aug;329(7462):371. DOI: 10.1136/bmj.38169.519653.EB
[10] Camargo RP, Simões JA, Cecatti JG, Alves VM, Faro S. Impact of treatment for bacterial vaginosis on prematurity among Brazilian pregnant women: a retrospective cohort study. Sao Paulo Med J. 2005 May;123(3):108-12. DOI: 10.1590/S1516-31802005000300004
[11] Daskalakis G, Papapanagiotou A, Mesogitis S, Papantoniou N, Mavromatis K, Antsaklis A. Bacterial vaginosis and group B streptococcal colonization and preterm delivery in a low-risk population. Fetal Diagn Ther. 2006;21(2):172-6. DOI: 10.1159/000089298
[12] Larsson PG, Fåhraeus L, Carlsson B, Jakobsson T, Forsum U; Premature study group of the Southeast Health Care Region of Sweden. Late miscarriage and preterm birth after treatment with clindamycin: a randomised consent design study according to Zelen. BJOG. 2006 Jun;113(6):629-37. DOI: 10.1111/j.1471-0528.2006.00946.x
[13] Svare JA, Schmidt H, Hansen BB, Lose G. Bacterial vaginosis in a cohort of Danish pregnant women: prevalence and relationship with preterm delivery, low birthweight and perinatal infections. BJOG. 2006 Dec;113(12):1419-25. DOI: 10.1111/j.1471-0528.2006.01087.x
[14] Hendler I, Andrews WW, Carey CJ, Klebanoff MA, Noble WD, Sibai BM, Hillier SL, Dudley D, Ernest JM, Leveno KJ, Wapner R, Iams JD, Varner M, Moawad A, Miodovnik M, O'Sullivan MJ, Van Dorsten PJ; National Institute of Child Health and Human Development, Maternal-Fetal Medicine Units Network. The relationship between resolution of asymptomatic bacterial vaginosis and spontaneous preterm birth in fetal fibronectin-positive women. Am J Obstet Gynecol. 2007 Nov;197(5):488.e1-5. DOI: 10.1016/j.ajog.2007.03.073
[15] Nejad VM, Shafaie S. The association of bacterial vaginosis and preterm labor. J Pak Med Assoc. 2008 Mar;58(3):104-6.
[16] Lee SE, Romero R, Kim EC, Yoon BH. A high Nugent score but not a positive culture for genital mycoplasmas is a risk factor for spontaneous preterm birth. J Matern Fetal Neonatal Med. 2009 Mar;22(3):212-7. DOI: 10.1080/14767050802616994
[17] McCormick KA, Moore SR, Siegel R; US Agency for Health Care Policy and Research, editors. Clinical Practice Guidelines Development: Methodological Perspectives. Rockville: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 1992. p. 115-27.
[18] Abrams P, Khoury S, Grant A. Evidence--based medicine overview of the main steps for developing and grading guideline recommendations. Prog Urol. 2007 May;17(3):681-4. DOI: 10.1016/S1166-7087(07)92383-0
[19] Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes; Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press (US); 2007. DOI: 10.17226/11622
[20] Elder HA, Santamarina BA, Smith S, Kass EH. The natural history of asymptomatic bacteriuria during pregnancy: the effect of tetracycline on the clinical course and the outcome of pregnancy. Am J Obstet Gynecol. 1971 Oct 1;111(3):441-62. DOI: 10.1016/0002-9378(71)90793-9
[21] Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, Berry SM. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998 Jul;179(1):186-93. DOI: 10.1016/S0002-9378(98)70271-6
[22] Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG 2nd, Rao AV. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995 Dec;333(26):1737-42. DOI: 10.1056/NEJM199512283332604
[23] Saling E, Fuhr N, Placht A, Schumacher E. Erste Ergebnisse der "Selbst-Vorsorge-Aktion von Schwangeren" zur Frühgeburtenvermeidung [Initial results of "preventive self-care by pregnant patients" for prevention of premature labor]. Arch Gynecol Obstet. 1995;257(1-4):178-85. DOI: 10.1007/BF02264816
[24] Martius JAH. Über die Bedeutung der Bakteriellen Vaginose und anderer Urogenitalinfektionen in der Schwangerschaft für die Frühgeburtlichkeit und die mütterliche peripartale Infektmorbidität [habilitation treatise]. Würzburg: Universität Würzburg, 1986.
[25] Eschenbach DA, Gravett MG, Hoyme UB, Holmes KK. Bacterial vaginosis during pregnancy. An association with prematurity and postpartum complication. In: Mardh PA, Taylor-Robinson D, editors. Bacterial vaginosis. WHO workshop on anaerobic curved rods and bacterial vaginosis. Stockholm: Almqvist and Wiksell; 1984. p. 213-8.
[26] McGregor JA, French JI, Seo K. Adjunctive clindamycin therapy for preterm labor: results of a double-blind, placebo-controlled trial. Am J Obstet Gynecol. 1991 Oct;165(4 Pt 1):867-75. DOI: 10.1016/0002-9378(91)90430-Y
[27] Donders GG. Bacterial vaginosis during pregnancy: screen and treat? Eur J Obstet Gynecol Reprod Biol. 1999 Mar;83(1):1-4.
[28] Hill GB. Preterm birth: associations with genital and possibly oral microflora. Ann Periodontol. 1998 Jul;3(1):222-32. DOI: 10.1902/annals.1998.3.1.222
[29] Viehweg B, Junghans U, Stepan H, Voigt Th, Faber R. Der Nutzen vaginaler pH-Messungen für die Erkennung potentieller Frühgeburten [Usefulness of vaginal pH measurements in the identification of potential preterm births]. Zentralbl Gynakol. 1997;119 Suppl 1:33-7.
[30] Andres FJ, Parker R, Hosein I, Benrubi GI. Clindamycin vaginal cream versus oral metronidazole in the treatment of bacterial vaginosis: a prospective double-blind clinical trial. South Med J. 1992 Nov;85(11):1077-80. DOI: 10.1097/00007611-199211000-00006
[31] Gorlero F, Macciavello S, Paccagnella F, Ferraiolo A, Gianrosso G, de Gecco L, Schito GC. Clindamycin phosphate vaginal cream - a local approach to the treatment of bacterial vaginosis. Report of the 3rd International Symposium on Vaginitis/Vaginosis; Madeira. Oxford: Oxford Chemical Communications; 1994.
[32] Hillier S, Krohn MA, Watts DH, Wolner-Hanssen P, Eschenbach D. Microbiologic efficacy of intravaginal clindamycin cream for the treatment of bacterial vaginosis. Obstet Gynecol. 1990 Sep;76(3 Pt 1):407-13.
[33] Dennemark N, Meyer-Wilmes M, Schlüter R. Screening and treatment of bacterial vaginosis in the early second trimester of pregnancy: a sufficient measure for prevention of preterm deliveries? Intern J STD AIDS 1997; 8(1):38–40. DOI: 10.1258/0956462971919435
[34] Lamont RF, Nhan-Chang CL, Sobel JD, Workowski K, Conde-Agudelo A, Romero R. Treatment of abnormal vaginal flora in early pregnancy with clindamycin for the prevention of spontaneous preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011 Sep;205(3):177-90. DOI: 10.1016/j.ajog.2011.03.047
[35] Bretelle F, Fenollar F, Baumstarck K, Fortanier C, Cocallemen JF, Serazin V, Raoult D, Auquier P, Loubière S. Screen-and-treat program by point-of-care of Atopobium vaginae and Gardnerella vaginalis in preventing preterm birth (AuTop trial): study protocol for a randomized controlled trial. Trials. 2015 Oct;16:470. DOI: 10.1186/s13063-015-1000-y




