Prostate biopsy related infection – Risk factors, prevention strategies and management approaches
Patrick N. Harris 1
Michael Holmes 2
Jeremy Grummet 3
Matthew Roberts 4
1 School of Medicine, The University of Queensland, Brisbane, Australia
2 Urology Department, Waikato Hospital, Hamilton, New Zealand
3 Department of Urology, Alfred Health, Melbourne, Australia
4 Faculty of Medicine, The University of Queensland, Brisbane, Australia
Abstract
Infectious complications following prostate biopsy are reported to be increasing in incidence with associated individual and public health burden. Complication rates parallel a rise in antibiotic resistant colonisation of rectal flora in patients undergoing biopsy. Following systematic review, risk factors for post biopsy infection, preventative strategies, alternative sampling methods, and management of infectious complications are presented. Risk factors for infection should be identified; including urogenital infection or antibiotic use, international travel, hospital exposure, bacteriuria, or previous transrectal biopsy. Patients with risk factors may benefit from an adjusted biopsy protocol-comprising transrectal biopsy under targeted prophylaxis, and/or the use of rectal disinfection techniques as well as a transperineal biopsy approach. Management of post biopsy infection should be based on individual risk, local resistance profiles, and in collaboration with infectious diseases specialty physicians.
Summary of recommendations
| Recommendation | LoE | GoR | |
|---|---|---|---|
| 1. The proportion of patients undergoing TRUS biopsy harbour antibiotic-resistant bacteria in their gut flora is not insignificant. Routine quinolone-based prophylaxis may no longer be sufficient for all patients. | 1B | A | |
| 2. Risk factors should be identified for all patients scheduled for prostate biopsy to determine if an altered prophylaxis regime is to be considered. These include: | 2A | B | |
| Urogenital infection and/or antibiotic use in last 6 months | 2A | ||
| International travel in last 6 months | 2A | ||
| Hospital admission or exposure (healthcare worker) in last 6 months | 2A | ||
| Current bacteriuria/indwelling catheter | 2A | ||
| Previous TRUS biopsy | 2A | ||
| Planned saturation biopsy | 2B | ||
| 3. Patients without risk factors may proceed to TRUS biopsy using quinolone-based prophlyaxis following informed consent of their low risk of sepsis, as well as clear instruction to seek urgent medical attention if they develop symptoms of infection. | 1B | A | |
| 4. Patients with risk factors should prompt the clinician to consider: | |||
| A transperineal biopsy, requiring only single dose prophylaxis with IV cephazolin, with risk of sepsis less than 1/1,000, OR | 2A/3 | B | |
| TRUS biopsy following rectal culture and targeted antibiotic prophlyaxis according to culture results, AND/OR | 2A | B | |
| TRUS biopsy with rectal disinfection using Povidone-iodine | 2A | B | |
1 Introduction
Transrectal ultrasound-guided (TRUS) biopsy of the prostate (TRUBP) is the most commonly used modality to diagnose prostate cancer resulting in millions of biopsies performed internationally each year. Widespread use of PSA testing, an ageing population and increasing implementation of active surveillance protocols for low risk disease led to more TRUS biopsies performed over the last two decades. While the U.S. Preventative Services Task Force recommendation in 2012 has resulted in reduced PSA testing and biopsy rates [1], TRUBP are still performed in high numbers worldwide. TRUBP is generally considered a safe procedure performed using local anaesthetic with or without procedural sedation, but infectious complications can occur; including urinary tract infection (UTI), prostatitis, and sepsis [2], [3]. These are due to inoculation of the prostate and surrounding blood vessels with bacterial flora of the rectal mucosa, particularly Gram-negative Enterobacteriaceae such as Escherichia coli. Sepsis occurs in approximately 1% of biopsy procedures and UTI in more than 6%, resulting in substantial health and economic burden [4], [5]. TRUBP is therefore considered a ‘contaminated’ procedure under European Association of Urology (EAU) guidelines, necessitating antibiotic prophylaxis as a standard of care for all cases [6], [7], [8], [9], [10]. Fluoroquinolone-based antimicrobial prophylaxis is recommended by many authorities, including the EAU and the American Urological Association, due to their broad coverage against rectal flora and good penetration into the prostate gland [11]. Duration of prophylaxis is varied between urologists and health services, with no evidence to suggest prolonged duration translates to reduced complications [7], [12], [13].
Despite prophylaxis, observational studies have reported increasing rates of infectious complications over the past two decades and postulate a strong association with changing patterns of antimicrobial resistance [14], [15], [16], [17]. The most clinically significant emerging phenotype is that of fluoroquinolone resistance, which has been reported to affect complication rates for other surgical procedures [4]. Teillant and colleagues have reported that, in the USA, 13,120 post-TRUBP infections per year are attributable to fluoroquinolone resistance, which would increase to 64,000 infections per year in the event of 100% fluoroquinolone resistance [4]. The management of TRUBP complications causes significant financial burden on health systems, reported to cost more than that due to methicillin-resistant Staphylococcus aureus and Clostridium difficile in the UK [18], [19]. The non-financial, unmeasurable burden of disease from TRUBP complications, including psychological burden of significant illness, hospital admission and anxiety regarding future biopsies, must also be considered [20].
In this chapter, we sought to systematically review and critically appraise available published literature on risk factors, prevention and management of TRUBP-associated infectious complications in order to provide recommendations for general use in daily urology practice.
2 Methods
A systematic search of the literature was conducted in January 2016 in accordance with the PRISMA statement and Cochrane Guidelines [21]. The Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, EMBASE, and LILACS databases were searched for the following key terms: prostat*, biopsy, infect*, culture*, bacter*, sepsis, fever, UTI. Only peer reviewed manuscripts were considered were inclusion.
A total of 4,494 citations were identified, which after exclusion of duplicates and screening by title and abstract, approximately 600 were considered for full text review. Review of reference lists of included manuscripts for applicable studies was also performed.
Studies were rated according to the level of evidence (LoE) and the grade of recommendation (GoR) using a system used in the EAU guidelines (2015) modified from the Oxford Centre for Evidence-based Medicine [22]. Overall, included studies were contained limited randomised data for most scenarios, and consequently the LoE is mostly 2A/2B and GoR B.
3 Results
3.1 Incidence
Complications following TRUBP are reported with great variability and subject to a lack of complication-specific standardised definitions. Furthermore, the incidence of complications varies according to the geographic region in which studies are conducted. Across published reports, a wide-ranging incidence of emergency department presentations (0–6%), hospitalisation (up to 4%), and severe sepsis of 0–1% is observed [3], [23], [24], [25]. In an attempt to standardise complication estimates across three key measures, hospitalisation, sepsis and acute urinary retention, Bennett and colleagues performed a systematic review and meta-analysis utilising directly standardised prevalence estimates based on cases of new prostate cancer cases according to GLOBOCAN [5]. The reported estimates are presented in table 1.
|
TRUBP Complication |
Global estimate |
|||
|
Continent |
Hospitalisation |
Sepsis |
Acute urinary |
|
|
Asia |
2.2% (0–7.7) |
1.0% (0.3–2.0) |
1.2% (0.2–6.8) |
40% (33–47%) |
|
Europe |
0.9% (0.1–4.7) |
0.7% (0–2.9) |
0.5% (0.4–4.0) |
22% (22–25%) |
|
North America |
0.8% (0.2–1.7) |
0.8% (0.4–5.7) |
0.2% (0.1–6.8) |
16% (13–18%) |
|
All figures expressed as pooled estimate % (95% confidence interval) |
||||
Many recent reports highlight an increasing incidence of TRUBP-related complications with time in parallel with a worldwide trend of increasing antimicrobial resistance and subsequent infection with fluoroquinolone resistant micro-organisms [17], [26], [27], [28], [29]. Despite this trend, 30-day mortality estimates remain between 0.1–1%, lower than the age adjusted population [14], [15], [16], [17], [26], [30], [31]. As fluoroquinolones are the predominant antimicrobial used for TRUBP prophylaxis, estimates of fluoroquinolone resistance have been included in table 1.
3.2 Risk factors
An appreciation for risk factors predictive of post-TRUBP infection allows the treating urologist to guide prophylaxis, as well as assist in patient selection for alternative sampling methods [33]. Reported risk factors for post-TRUBP infection are listed in table 2.
|
Host related |
Rectal flora antimicrobial resistance (fluoroquinolone most commonly) |
|
Recent urogenital infection and/or antibiotic use |
|
|
Hospital admission or exposure (healthcare worker) |
|
|
Recent international travel |
|
|
Bacteriuria (pre-biopsy urine culture, indwelling catheter in situ) |
|
|
Co-morbidities (Diabetes mellitus, cardiac valve replacement, chronic obstructive pulmonary disease, benign prostatic hyperplasia) |
|
|
Surgeon related |
Approach – transrectal, transperineal, MRI-guided |
|
Repeat biopsy |
|
|
Greater number of biopsy cores |
|
|
Contaminated ultrasound gel |
3.2.1 Host-related
3.2.1.1 Antimicrobial resistance
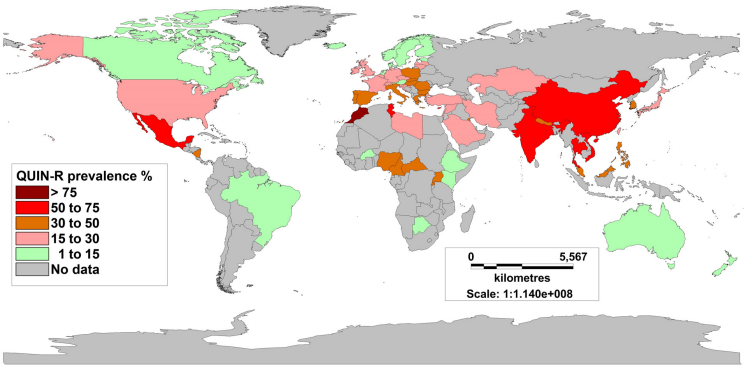
With fluoroquinolone therapy being most commonly used for TRUBP prophylaxis, the risk factor most predictive of post-TRUBP infection is fluoroquinolone resistance in rectal flora [16], [17], [23], [24], [28], [30], [34], [35]. TRUBP causes translocation of rectal bacteria across the rectal mucosa into the prostate and bloodstream. The mechanism of antimicrobial resistance development in rectal flora is induced by selection pressure following fluoroquinolone use, or acquired by travel to areas of high endemic antimicrobial resistance [3], [28], [36], [37], [38]. Fluoroquinolone resistance in E. coli blood stream isolates has been reported to average 12% in the United States and 20% in Europe, with known fluctuation between 10 and 45% secondary to regional differences [3]. The prevalence of fluoroquinolone resistance has been observed to be higher in Asian countries (26.7–92%) [39], [40].
A recent meta-analysis, reporting on nine studies and 2,541 patients, reported that prevalence of fluoroquinolone resistance in rectal flora may be higher (20.4% vs. 12.8%) after fluoroquinolone therapy prior to TRUBP. There was a higher incidence of TRUBP-associated infections in patients with fluoroquinolone resistant rectal cultures compared with fluoroquinolone sensitive (7.1% vs. 1.1%), which translated to a 7.4% vs. 1.4% risk difference, respectively. Thus, it was estimated that for every 14 men with a fluoroquinolone resistant rectal culture, one additional TRUBP-associated infection was observed compared with men displaying fluoroquinolone-sensitive cultures [35].
These findings were supported by a collaborative analysis of the original source data, with fluoroquinolone resistance associated with an increased overall risk of infection (OR 3.98, 95% CI 2.37–6.71) and hospitalisation (OR 4.77, 95% CI 2.50–9.10), which were highest with fluoroquinolone monotherapy [41]. Further confirmation in independent cohorts further support a causative relationship between TRUBP-associated infections and fluoroquinolone resistance, however are based on non-randomised data [42], [43], [44].
3.2.1.2 Prior urogenital infection and/or antibiotic use
A number of studies in patients undergoing TRUBP have reported antimicrobial use within the past 3–6 months to be significantly associated with fluoroquinolone resistant carriage in the rectal flora [17], [36], [45], [46], [47], [48]. These findings have been corroborated using meta-analysis, with history of genitourinary infection (OR 2.56; 95% CI 1.13–5.79; n=1,218) and prior fluoroquinolone use (OR 4.12; 95% CI 2.30–7.37; n=1,356) reported to be significant risk factors for fluoroquinolone-resistance colonisation. The use of antimicrobials in these scenarios causes selection pressure on the normal rectal flora, resulting in preferential growth of resistant species [49]. Wagenlehner and colleagues demonstrated on rectal swab culture that single dose prophylaxis was sufficient to select for ciprofloxacin resistant organisms, with a four-fold increase in fluoroquinolone resistance after administration [50]. This has also been demonstrated in studies investigating empiric antibiotics for elevated PSA, with extended antibiotic administration leading to significantly higher rates of sepsis and resistance following biopsy [51]. Given the high concordance between fluoroquinolone resistance and extended-spectrum beta-lactamase (ESBL) production, it is unsurprising that the use of fluoroquinolone prophylaxis has also been shown to co-select for ESBL-producing E. coli [52].
3.2.1.3 Hospital admission or exposure (healthcare worker)
Hospitalisation in the year preceding biopsy has also been shown to increase carriage of fluoroquinolone resistant organisms and increase biopsy related infection [11], [17], [46], [53]. Interestingly, this risk has also been observed in physicians [54], as well as relatives of hospital employees [55].
3.2.1.4 Recent international travel
International travel, particularly involving contact with healthcare facilities, also increases carriage of resistant organisms [36], [48]. This was particularly true of exposure to healthcare facilities and water sources in the Indian subcontinent and South-East Asia, where resistance rates are known to be high [5], [38], [56].
3.2.1.5 Bacteriuria (pre-biopsy urine culture, indwelling catheter in situ)
Asymptomatic bacteriuria is an established risk factor and routine testing is recommended in the EAU guidelines, though poor compliance with this recommendation is reported [57], [58]. History of urethral catheterisation or prior urogenital infection (urinary tract infection or prostatitis) is also associated [31], [45], [59].
3.2.1.6 Co-morbidities
The presence of co-morbidities such as diabetes mellitus, cardiac valve replacement, chronic obstructive pulmonary disease, immunosuppression, or benign prostatic hyperplasia have been variably reported to increase the risk of post-TRUBP complications. Higher comorbidity scores have also been associated with a significantly increased risk of hospitalisation post-biopsy in multiple large retrospective cohorts [14], [31], [60]. Diabetes and the metabolic syndrome have been reported to be associated with both increased risk of infectious complications, and carriage of resistant organisms [15], [31], [61], [62], [63]. However, on meta-analysis of available risk factors, diabetes (OR 1.37; 95% CI 0.77–2.46; n=1,140) was not significantly associated with fluoroquinolone-resistant colonisation [35].
3.2.1.7 Compliance
Non-compliance is difficult to reliably assess but may contribute to complication rates in populations with a relatively low baseline prevalence of fluoroquinolone resistance. For instance, Suwantarat and colleagues reported a 43% post-TRUBP infection rate in patients not presenting for rectal swab and thus assigned to empiric prophylaxis, despite an underlying prevalence of fluoroquinolone resistance of 16% [64]. Of greater concern, the compliance of the treating urologist to best practice guidelines can influence sepsis outcomes, with Womble and colleagues reporting that patients on non-compliant regimens were more likely to be hospitalised following TRUBP (3.8 vs. 0.89%) [24]. These findings were supported by a large multicenter study by Bruyere and colleagues reported noncompliance with antibiotic prophylaxis guidelines to be a risk factor for post-TRUBP sepsis (OR 2.3, 95% CI 1.4–3.9) [45].
3.2.2 Surgeon related
3.2.2.1 Mode of biopsy
Transperineal biopsy is an alternative method of sampling providing transcutaneous access to the prostate. Prostate cancer detection rates have been reported to be similar, though some investigators contend that the transperineal approach can better detect anteriorly sited tumours [65], [66]. Transperineal sampling allows thorough skin preparation in line with typical surgical procedures, and prophylactic antibiotics (eg cephazolin) are targeted to skin flora and common urinary pathogens [67], [68]. As transperineal biopsies avoid the rectum, this approach has traditionally been thought to have lower rates of infection than the ‘transfaecal’ route of TRUBP. Transperineal biopsy has been classified as a ‘clean-contaminated’ procedure in the EAU guidelines, however it could even be argued that it is ‘clean’ as there is often no breach of urinary tract mucosa using this approach [10]. This benefit is less clear in practice, and studies with direct comparison of morbidity between transrectal and transperineal biopsy are lacking.
Recent large prospective investigations have sepsis rates approaching zero for transperineal biopsy. In a prospective study of 2,086 patients from Japan no patient required additional therapy for infectious complications; and another prospective report from China, one patient out of 3,007 developed urosepsis post biopsy [69], [70]. No cases of urosepsis were seen in a prospective series of 3,000 patients from Italy [71].
Transperineal biopsy has clear benefits in decreasing infection related morbidity, but is not without drawbacks. It is logistically more involved and time-consuming, requiring admission to hospital, an operating theatre, and usually general anaesthesia. Transperineal biopsy is also associated with higher rates of post-procedure urinary retention [5].
| TRUS biopsy | Transperineal biopsy | ||
|---|---|---|---|
| Complication | Standardised prevalence (95% CI) |
Standardised prevalence (95% CI) |
|
| Acute Urinary Retention | Total | 0.9% (0–3.6) | 4.2% (0.2–12.9) |
| Asia | 1.2% (0.2–6.8) | 1.78% (0–7.5) | |
| Europe | 0.5% (0.4–4.0) | 2.6% (0–11.3) | |
| North America | 0.2% (0.1–6.8) | 6.2% (0.1–25.6) | |
| Hospitalisation | Total | 1.1% (0–3.9) | 0.9% (0–3.4) |
| Asia | 2.2% (0–7.7) | 0.6% (0.1–1.4) | |
| Europe | 0.9% (0.1–4.7) | 1.0% (0.1–5.0) | |
| North America | 0.8% (0.2–1.7) | 1.0% (0.2–2.1) | |
| Sepsis | Total | 0.8% (0–3.0) | 0.1% (0–0.2) |
| Asia | 1.0% (0.3–2.0) | 0.0% (0–0.5) | |
| Europe | 0.7% (0–2.9) | 0.1% (0.1–0.5) | |
| North America | 0.8% (0.4–5.7) | 0.2% (0.1–0.7) |
3.2.2.2 Number of cores
The extent of sampling has also been a target for risk reduction. An ‘extended’ biopsy strategy of 12–18 cores is currently recommended to optimise cancer detection, and does not increase complications compared to sextant biopsy [72], [73]. Biopsies of >18 cores do however have a poor side-effect profile and so called ‘saturation’ biopsies (>20 cores including transition zone) are rarely indicated [72], [74]. 18-gauge needles are the most widely used for sampling, and produce similar specimen quality to 16- and 14-gauge needles with low morbidity [75]. Local anaesthetic administration has also not been associated with increased infectious complications [45].
3.2.2.3 Previous biopsies
Repeat biopsies are indicated in men with persistent suspicion of prostate cancer according to elevated PSA, abnormal DRE or suspicious appearance on imaging [76]. Reports regarding the association between repeat biopsies and an increased risk of infectious complications compared with initial biopsies are mixed [45], [77], [78]. Repeat biopsies are an integral part of active surveillance, a practice commonly used in low grade low volume prostate cancer [76]. Any potential risk is concerning in this context, with a retrospective analysis reported increased odds of an infection (OR 1.33, 95%CI 1.01– 1.74) for every previous biopsy in 591 consecutive men undergoing TRUBP [78].
Repeat biopsy has been reported to be a risk factor for colonisation with resistant E. coli strains [79], with a progressive increase reported for each biopsy undertaken, specifically a 22.8% baseline resistance, not associated with biopsy history, while for men with initially fluoroquinolone sensitive swabs, a progressive increase in prevalence of 10.6% was observed at each of second and third biopsies [42]. Meta-analysis of available data (OR 0.92; 95% CI 0.68–1.25; n=1,615) did not support this association [35].
Table 4 presents a risk assessment questionnaire, based on available data, to aide clinicians in assessing for fluoroquinolone resistance and subsequent risk of post-TRUBP complication.
|
Rectal flora antimicrobial resistance |
|
|
Antibiotic use (especially fluoroquinolone)? |
|
|
Recent hospital admission? |
|
|
Occupation as healthcare worker? |
|
|
Recent international travel (especially South-east Asia or South America)? |
|
|
Bacteriuria |
Pre-biopsy urine culture indicated? |
|
Indwelling catheter in situ? |
|
|
Co-morbidities |
Diabetes mellitus? |
|
Cardiac valve disease/replacement? |
|
|
Chronic obstructive pulmonary disease? |
|
|
Benign prostatic hyperplasia? |
|
|
Other immunosuppressive disorder or treatment? |
|
|
Previous biopsy |
Previous biopsy? How many? |
3.3 Prevention strategies
3.3.1 Antimicrobial prophylaxis – empiric versus culture-directed (targeted)
An evolving body of evidence supports either an expanded antibiotic protocol or one targeted to rectal cultures. Expanded antibiotic protocols can consist of either a broad spectrum antibiotic or the use of multiple antibiotics. While initial studies cultured isolates on non-selective agar, most studies perform selective culturing on fluoroquinolone-impregnated MacConkey agar plates [80]. The fluoroquinolone concentrations can vary between 1 mg/L and 10 mg/L, often corresponding with pathogen minimum inhibitory concentration (MIC) estimates [35].
Targeted prophylaxis aims to remove antimicrobial resistance as a contributor to post-TRUBP infection. To date, five North American studies have prospectively used this principle of targeted therapy to reduce TRUBP-associated infections [64], [81], [82], [83], [84]. In the absence of high quality randomised data, these matched results were combined using meta-analysis (n=2,302). Patients using empirical prophylaxis demonstrated higher (3.1%, 95% CI 2.1–4.1%) infection rates than those using targeted methods (0.6%, 95% CI 0–1.5%). These updated estimates are similar to those calculated by Roberts and colleagues using unmatched cohorts (n=2,786), which were 3.3% (95% CI 2.6–4.2%) and 0.3% (95% CI 0–0.9%) respectively [35]. When stratified according to fluoroquinolone resistance status (resistant versus sensitive), the greatest risk difference was observed for empiric prophylaxis (7.8%, 95%CI 3.7–12.0%) compared with targeted prophylaxis (1.6%, 95%CI –0.8–4.0%). When applied to patients with fluoroquinolone sensitive rectal cultures, either passively using empiric broad-spectrum prophylaxis or actively using targeted prophylaxis, this equated to a number needed to treat (NNT) of 13 (95%CI 9–28). In contrast, Liss and colleagues analysed a multicenter retrospective database of over 5,000 patients in which 34% received targeted prophylaxis, and observed no difference in complications between targeted and empiric prophylaxis groups [34].
A major criticism of targeted prophylaxis is the unknown cost-benefit estimates, incorporating the extra resources required for this prophylaxis method. Taylor and colleagues estimated that targeted prophylaxis resulted in a cost saving of US$4,499 per TRUBP infectious complication averted [81]. Similarly, Duplessis and colleagues reported the cost of rectal culturing of US$350 per 100 patients (US$2.10 per culture) was less than the ~US$15,000 per 100 patients in the historical control group [82]. Suwantarat reported an estimated overall cost of US$30 per culture.
In addition to potentially reducing complication rates, targeted prophylaxis also serves to facilitate antimicrobial stewardship, as supported by Liss and colleagues [34]. Limiting absolute targeted therapy to narrow spectrum agents is prostatic tissue penetration, which is highest for fluoroquinolones [3], trimethoprim [85] and fosfomycin trometamol [86]. Thus, targeted methods may help to reduce the burden of TRUBP on antimicrobial resistance.
3.3.2 Decontamination
While post-TRUBP complications persist in the face of spreading antimicrobial resistance as well as infection despite adequate antimicrobial prophylaxis, adjunct strategies of ‘decontamination’ prior to biopsy warrant consideration. Strategies including bowel preparation and disinfection of the rectal mucosa are aimed at reducing the bacterial load involved in the inherently ‘dirty-to-clean’ passage of the TRUBP biopsy needle. Decontamination strategies for TRUBP biopsy are inconsistently practiced and reported less compared to antimicrobial-related studies [12], [87].
3.3.2.1 Rectal disinfection
With any transcutaneous intervention, from phlebotomy to major surgery, preparation and disinfection of the skin prior to incision is a worldwide standard of care. The skin preparation agent Povidone-iodine rectal preparation (PIRP) is simple and affordable, not associated with selection of resistant bacteria, and proven safe for colorectal surgery [88]. From seven controlled trials, including 2,049 patients, of rectal disinfection using PIRP prior to TRUBP, significant reductions in fever, bacteriuria and bacteraemia (RR 0.31; 95% CI 0.21–0.45) regardless of prophylaxis used have been reported. PIRP was shown to be superior to antimicrobials in preventing bacteraemia (RR 0.38; 95% CI 0.16–0.90), while a combination of PIRP and antibiotics reduced fever (RR 0.11; 95% CI 0.02–0.85) and bacteraemia (RR 0.25; 95% CI 0.08–0.75) [89]. Outside of this meta-analysis, PIRP has been reported to significantly reduce TRUBP-associated infections (0 vs. 1.8%) and colony counts determined by rectal swab culture (by up to 97%) [90]. However, a randomised controlled trial of prophylactic povidone-iodine use demonstrated insignificantly reduced complication rates (2.6%) compared with control (4.5%), in a study that is likely to have been underpowered [91]. The optimal method of administering PIRP has not been fully eluciadated but the use of a suppository or gauze soaked in povidone-iodine has been reported to be superior to a rectal enema [89], [92]. Chlorhexidine as a rectal preparation agent is currently under investigation [93].
3.3.2.2 Rectal cleansing
Available evidence regarding preparation with a rectal cleansing enema (e.g. Fleet sodium phosphate) is sparse due to implementation by a minority (18–30%) of urologists [13], [94]. Enema use has been demonstrated to reduce rates of bacteraemia following TRUBP, with studies including a small RCT without antibiotic cover and meta-analysis of observational studies where prophylaxis was administered [95], [96] [97]. Other reports have concluded that enema increases patient discomfort without improving clinical outcomes [98]. A retrospective study of 1,438 patients found that Fleet enema administration made no significant difference to the incidence of infection post biopsy [29]. A recent prospective report has suggested rectal enema is equally as effective as full bowel preparation with polyethylene glycol [99]. Of note, limited contemporary data is available in the era of increasing resistant bacteria causing infectious complications.
3.3.2.3 Other approaches
Among novel methods under investigation, needle disinfection prior to each biopsy, using 10% formalin showed similar TRUBP-associated infection rates (0.3%) in 1,642 patients to a historical comparison without formalin disinfection (0.8%). Of further interest, growth of fluoroquinolone resistant E. coli exposed to formalin on MacConkey and blood agars was non-existent, with formalin exposure estimated to be safe [100].
3.3.3 Biopsy method
3.3.3.1 Transperineal biopsy
Recent reports suggest minimal sepsis rates with the transperineal procedure [2], [68], further supported by three large cohort studies totaling 8,093 patients with one case of urosepsis reported and recent meta-analysis estimate of 0.1% [5], [69], [70], [71].
Transperineal sampling has typically been reserved for patients at high risk of sepsis, or for repeat biopsies, especially those with a previous non-diagnostic TRUBP [2]. Nonetheless, preventing the translocation of faecal flora is increasingly important in the era of multi-resistant bacteria. From an antimicrobial stewardship perspective, transperineal biopsy may also avoid selecting for fluoroquinolone- or multi-resistant bacteria, and in particular stem the increasing reliance on an ever expanding range of antibiotics for biopsy prophylaxis.
3.3.3.2 MRI-Guided biopsy
Multi-parametric magnetic resonance imaging (mp-MRI) has emerged in recent years as a valuable tool in the diagnosis and monitoring of prostate cancer. MRI is used to guide biopsies using ‘cognitive’, ‘in-bore’, or MRI-ultrasound ‘fusion’ biopsy techniques [101]. Tissue diagnosis with MRI-guided biopsies is generally via the transrectal route, and preliminary experience suggests that complication rates are similar to the conventional TRUS approach. Urosepsis rates of 0–2% have been reported with both in-bore and fusion techniques [101]. Improved localisation with mp-MRI can reduce unnecessary biopsies, as well as the need for repeat biopsy in patients on active surveillance [101], [102], [103]. The general availability of MRI remains limited, and approximately 10% of significant lesions are ‘MRI-invisible’, so systematic cores remain necessary [103].
3.4 Management of prostate biopsy related infection
When considering the optimal treatment for a patient with an infectious complication following prostate biopsy, several factors need to be taken into account. This includes the severity of the clinical presentation (which may range from relatively mild cystitis or prostatitis to severe sepsis with multi-organ failure), the likelihood of resistance to empirical antibiotics, the co-morbidities of the host and whether anatomical complications co-exist (such as prostate abscesses or urinary tract obstruction). Choosing appropriate initial therapy is critical as these infections can progress quickly and may result in life-threatening complications. Inadequate or delayed empirical therapy has been associated with excess mortality in Gram-negative sepsis, especially in the setting of a high background prevalence of ESBL-producers [104], [105], [106]. Furthermore, inadequate empirical therapy is not uncommon in the setting of post-TRUBP sepsis, occurring in 36% of patients in one study [28]. The use of rectal culturing prior to TRUBP may help better inform initial antimicrobial selection and rationalise treatment options.
3.4.1 Initial assessment and risk of infection with a multi-drug resistant (MDR) organism
Obtaining a detailed history of recent antibiotic use may help assess the risk of resistance and, if fluoroquinolones have been used for prophylaxis, this class of drug should be avoided for empirical therapy. As noted previously, a significant risk factor for the likelihood of infection with a multi-drug resistant pathogen, is recent travel to a country highly endemic for Gram-negative resistance within the preceding 6 months [107]. The prevalence of resistance mechanisms such as ESBLs or carbapenemases in Gram-negative uropathogens varies widely across the world, and the situation is dynamic. In some high-prevalence countries resistance to 3rd generation-cephalosporins (a marker for ESBL production) in Enterobacteriaceae (such as E. coli or K. pneumoniae) can exceed 50% [32]. Resistance to carbapenems is less common but, driven by the recent emergence and spread of carbapenemase genes such as NDM, KPC or OXA-types, trends in some countries are alarming [32]. In Greece, for instance, more than 50% of K. pneumoniae isolates are now resistant to carbapenems [108], and for many parts of the world, reliable surveillance data are lacking [109].
Carbapenemase-producers tend to also possess numerous other resistance determinants, rendering them multi-drug resistant (MDR), extensively-drug resistant (XDR) or even pan-drug resistant (PDR) [110], [111]. Clearly this can dramatically reduce treatment options and makes selecting effective empirical therapy extremely problematic should these strains become predominant. In some patients, who are known to be colonised with MDR pathogens, alternatives to TRUBP or avoidance of any interventional procedure may have to be considered given the risks involved [112].
Risk prediction scores for assessing the likelihood of infections with an ESBL-producing organism in the context of Gram-negative sepsis have been developed for use in high-prevalence settings. In an Italian cohort, a scoring system based on recent hospitalisation, admission from another healthcare facility, a Charlson co-morbidity score of ≥4, recent beta-lactam or fluoroquinolone therapy, urinary catheterisation or age ≥70 years was predictive high risk for ESBL infection [113]. Such scoring systems have worked well in other settings with minor modifications [114], but require validation in a local context before they can be reliably implemented.
3.4.2 Early recognition of infectious complications
It is important for patients undergoing TRUBP to be made aware of the signs and symptoms of infection should they occur post procedure. Specifically, they should be advised to urgently seek medical attention if they experience fever, lethargy, difficulty passing urine, perineal pain, testicular swelling or dysuria.
The early recognition and effective treatment of sepsis is a key factor in improving patient outcomes, and management should broadly follow international guidelines, such as the Surviving Sepsis Campaign [115]. Recently, updated definitions have been developed to aid the early recognition of sepsis [116]. Previous definitions provided limited discrimination between sepsis and other causes of the systemic inflammatory response syndrome (SIRS). The updated 2016 consensus (“Sepsis-3”) definitions place emphasis upon the presence of end-organ dysfunction, using the sequential organ dysfunction assessment (SOFA) score, which can be easily calculated from a variety of clinical and laboratory values, with a score ≥2 associated with a 10% or greater risk of hospital mortality. A simplified bedside assessment tool termed quickSOFA (qSOFA) can be used as a rapid screen for sepsis, where at least 2 of the following criteria are present: respiratory rate ≥22/min, altered mentation, or systolic blood pressure ≤100 mm Hg [116], prompting further assessment and intervention. Within the context of sepsis, patients with septic shock can be identified by the presence of persistent hypotension requiring vasopressors to maintain mean arterial pressure ≥65 mm Hg and serum lactate level >2 mmol/L despite adequate fluid resuscitation. When such parameters are met, in-hospital mortality may exceed 40% [116].
3.4.3 Empirical therapy for infectious complications
Although Gram-positive organisms such enterococci may be implicated in post biopsy complications, Gram-negative bacteria such as E. coli are vastly more common and carry a greater risk of severe life-threatening infection. As such, empirical regimens must have adequate coverage to reflect local patterns of resistance in this species. Knowledge of the local epidemiology and rates of resistance in key uropathogens is essential for developing local guidelines for empirical therapy. Most microbiology laboratories can provide antibiogram data for urinary tract isolates to inform such decisions, or this information may be available from national surveillance data (e.g. [108]). An advantage for the routine use of pre-biopsy rectal screening (close to the date of biopsy) is that positive cultures can guide empirical therapy should infection occur.
Given the difficulty in reliably predicting susceptibility to empirical treatment regimens, it is critical that appropriate microbiological specimens are collected for culture, including a mid-stream urine and blood cultures, if the patient is febrile or shows other signs of sepsis. The results of these tests should also be rapidly available to the treating clinician should they reveal positive cultures.
In general, given the association with fluoroquinolone prophylaxis and MDR-E. coli infections, patients presenting with urinary sepsis post-TRUBP will require a broader spectrum of antibiotic coverage than patients with community-onset infections without prior healthcare exposure [6]. Therapy with agents such as 3rd generation cephalosporins (e.g. ceftriaxone or ceftazidime), ampicillin, quinolones or gentamicin may have a high likelihood of resistance in this context, especially in areas with an elevated prevalence of ESBL-producing E. coli or in patients exposed to such environments through travel. In the context of relatively low prevalence of resistance, options such as ceftriaxone or ampicillin and gentamicin may be adequate [117]. In other settings, broader-spectrum empirical options need to be considered. This could include, but is not limited to, piperacillin-tazobactam, carbapenems (e.g. ertapenem, imipenem or meropenem) or amikacin (usually in combination with a beta-lactam agent). Although aminoglycosides have several advantages for the treatment of urinary sepsis (rapid bactericidal effect, high concentration in the urine, a post antibiotic effect) toxicity concerns should generally limit their use for no more than 48 hours until a directed agent can be selected according to culture results [118].
3.4.4 Directed therapy for MDR Gram negative pathogens
Treatment guidelines for urinary infections often do not adequately address treatment options for MDR pathogens. Consultation with an infectious disease practitioner or medical microbiologist is recommended for these difficult-to-treat organisms. Reasons for this might include: the need for additional antimicrobial susceptibility testing for less common agents, treatment requiring the use of unfamiliar or potentially toxic agents and, in some circumstances, combination therapy may be warranted. Some agents that may test susceptible against highly resistant strains, may be ineffective in the context of urinary infections. For instance, tigecycline may be one of the agents that remains effective in vitro against XDR isolates, but penetrates poorly into the urinary tract and only achieves limited serum levels, and may be associated with excess mortality in the treatment of sepsis [119]. Hence, treatment of drug-resistant strains can be a challenge. Various therapeutic options are discussed below:
3.4.4.1 Carbapenems
For ESBL-producing Enterobacteriaceae, treatment with ampicillin, aztreonam, cephazolin, or third-generation cephalosporins (such as ceftriaxone or ceftazidime) will generally be ineffective [120]. For several reasons, carbapenems (such as meropenem, imipenem, doripenem or ertapenem) have been regarded as the treatment of choice for ESBL-producers [120], [121]. In vitro, carbapenems are generally stable to the hydrolytic effects of ESBLs [122] and other broad-spectrum beta-lactamases such as AmpC [123]. They are also less prone to inoculum effects and historically most urinary Gram-negative isolates have remained susceptible to this class of drug [120]. However, carbapenem resistance has been increasing in many parts of the world [32].
Observational studies have supported the use of carbapenems for infections caused by ESBL-producers. Poor outcomes have been demonstrated in bacteraemic patients treated with non-carbapenems drugs (especially cephalosporins or quinolones) [124], [125], [126], [127]. In a meta-analysis of studies comparing carbapenems with cephalosporins, quinolones or other alternatives (except beta-lactam/beta-lactamase inhibitor drugs such as piperacillin-tazobactam), the pooled RR for mortality when carbapenems were used for definitive therapy was 0.65 (95% CI: 0.47–0.91) and 0.50 for empirical therapy (95% CI: 0.33–0.77) [127].
Given the rise in carbapenem resistance around the world, it has been increasingly necessary to consider alternatives to carbapenems, as excess carbapenem use is likely to be a key driver for resistance. This has given rise to some reconsideration of drugs that were previously considered less effective, including drugs such as cefepime, beta-lactam/beta-lactamase inhibitor (BLBLI) drugs like piperacillin-tazobactam and amoxicillin-clavulanate, or older agents such as fosfomycin, pivmecillinam or temocillin.
3.4.4.2 Cefepime
ESBL-producers may often test resistant to cefepime, but not invariably; for ‘susceptible’ isolates there is some uncertainty as to whether cefepime remains an effective option. It is not stable to the hydrolytic activity of ESBLs and is subject to inoculum effects in vitro [128] – whereby the MIC significantly increases in the presence of a heavy bacterial load. A retrospective study found the use of cefepime compared to carbapenems to treat bacteraemia caused by ESBL-producers was independently associated with increased 30-day mortality on multivariate analysis (OR 9.9; 95% CI 2.8–31.9) [129]. However, some authors have suggested that if the organism MIC is low (e.g. ≤1 mg/L by European Committee on Antimicrobial Susceptibility Testing standards cefepime may remain effective, but higher or more frequent dosing may be necessary if the MIC falls in a higher range but below the breakpoint for resistance (e.g. MIC range 2–4 mg/L) [130]. In general, cefepime should probably be reserved as a treatment option against ESBL-producers only in specific circumstances, under specialist advice and when other options are unavailable. In contrast, cefepime remains stable to other broad-spectrum beta-lactamases such as AmpC – a chromosomally encoded and inducible enzyme found in some species such as Enterobacter cloacae or Citrobacter freundii [123]. Some observational studies suggest that cefepime remains an effective treatment for AmpC-producers [131], [132]. In a meta-analysis of 8 observational studies comparing mortality following the use of cefepime or carbapenems for the treatment of bloodstream infection caused by AmpC-producers such as Enterobacter spp., there was no significant difference in outcome (OR 0.61; 95% CI 0.27–1.38), even after adjustment for potential confounders (adjusted OR 0.59; 95% CI 0.14–2.52) [133]. Patients with bloodstream infections caused by Enterobacter spp. with MICs in the range of 4–8 mg/L (the new ‘susceptible dose dependent’ [SDD] category defined by the Clinical and Laboratory Standards Institute), experienced worse outcome in one study [134]. Some concerns over the efficacy of cefepime for the treatment of sepsis arose following meta-analyses that suggested cefepime may be associated with a small but significantly increased risk of death (RR 1.26; 95% CI 1.08–1.49) [135], although a larger analysis by the FDA, including patient-level data, found no statistically significant increased risk of mortality [136]. These findings are complicated by the fact that the original dosing regimens for cefepime may have been set too low or infrequently (e.g. 1 g 12-hourly), whereas higher dosing (e.g. 2 g 8-hourly) may be necessary to achieve adequate pharmacokinetic-pharmacodynamic targets, especially in severe infections with organisms showing elevated MICs [137].
3.4.4.3 Beta-lactam/beta-lactamase inhibitors (BLBLIs)
By definition, ESBL enzymes are inhibited in vitro by beta-lactamase inhibitors such as clavulanate or tazobactam. Although susceptibility to BLBLIs may vary considerably across the world and by species (ESBL-E. coli are generally more likely to be susceptible to piperacillin-tazobactam than ESBL-K. pneumoniae), a significant proportion of ESBL-producers remain susceptible to piperacillin-tazobactam, or even amoxicillin-clavulanate [138]. However, until recent years, there were limited clinical data to support their use as therapy against ESBL-producers.
Concerns over inoculum effects in infections with a high bacterial burden [139], the presence of multiple beta-lactamases that may not be well inhibited by the inhibitor component [140] and some animal model data suggesting worse outcomes when compared with carbapenems, especially with ‘high inoculum’ infections [141] limited enthusiasm for their clinical use. In recent years this concept has been questioned in light of some observational studies that suggest that BLBLIs may be non-inferior to carbapenems for treating ESBL-producers, and may reduce the risk of subsequent colonisation or infection with other MDR pathogens [138], [142], [143], [144]. A meta-analysis of 21 observational studies reporting mortality outcome for patients with bacteraemia caused by ESBL-producers demonstrated that non-inferior outcomes were seen with patients treated with BLBLIs compared with carbapenems either for empirical (RR 0.91; 95% CI 0.66–1.25) or definitive (RR 0.52; 95% CI 0.23–1.13) therapy [127]. This meta-analysis used crude mortality, unadjusted for co-morbidity, which will tend to bias against carbapenems – which may be given in ‘sicker’ patients. A recent well-designed observational study, which adjusted for the propensity to prescribe carbapenems, suggested that empirical piperacillin-tazobactam may be associated with increased mortality when used for treating bacteraemia caused by ESBL-producers [145]. However, in the largest international observational cohort of patients with bacteraemia caused by ESBL-producers, BLBLIs demonstrated equivalent efficacy to carbapenems when used either for empirical or directed therapy [146]. These discrepant findings reflect the difficulties in drawing conclusions from observational studies. It is worth noting that no randomised controlled trials have yet been reported that directly compare carbapenems with alternatives for ESBL-infections, although such studies are being undertaken [147], [148]. In the meantime, piperacillin-tazobactam is likely to be a reasonable carbapenem-sparing option for treating ESBL-producers that test susceptible in vitro, especially from a urinary tract source in a patient without features of septic shock or multi-organ dysfunction.
3.4.4.4 Fosfomycin
Fosfomycin has been used for many years as an effective single dose therapy for uncomplicated UTI, and is now part of international guidelines for this indication [149]. In recent years, it has been re-evaluated as a useful therapy for infections caused by ESBL or AmpC-producing Enterobacteriaceae. Probably because it has not been widely used, resistance rates tend to be low, even amongst MDR strains [150], [151]. It has shown broadly similar efficacy in comparison to carbapenems for patients with lower tract infections caused by ESBL-producers, including for patients with complicating factors such as catheterisation or recent prostate surgery [152]. Although published experience with using fosfomycin for treating infections post TRUBP are sparse, it is notable that fosfomycin appears to achieve adequate prostate tissue levels and may be an option for prophylaxis in patients known to be colonised with MDR Gram-negative pathogens [86], [153]. However, as uncertainty exists over the ability of the drug to adequately penetrate renal tissue, guidelines have suggested alternatives to oral fosfomycin for the treatment of pyelonephritis [154]. While fosfomycin is usually available orally, but intravenous formulations do exist which may be difficult to access in some countries. When treating serious MDR infections with few other treatment options, intravenous fosfomycin may be an effective option. It has been reported to have successfully treated a complex MDR-E. coli infection post TRUBP with prostatic and metastatic multifocal abscesses [20]. There are also case reports of successful therapy for prostatitis [155].
3.4.4.5 (Piv)mecillinam
Mecillinam is an aminidopenicillin with a broad range of activity against Gram-negative pathogens. However, given its poor oral bioavailability, the pro-drug pivmecillinam is used. It is now recommended in the IDSA guidelines for the treatment of uncomplicated UTI in women [149], although there are some concerns for its efficacy in more complex urinary infections.
It has the advantage of stability to ESBL and AmpC enzymes and retains good activity against most ESBL-producing E. coli [156]. It is another ‘rediscovered’ antibiotic that has been available for use in Europe for many years, but is not widely employed or licensed elsewhere. In vitro, at least, it appeared effective against ESBL-producing E. coli, especially when combined with the beta-lactamase inhibitor clavulanate [157]. There are no published data with respect to pivmecillinam treatment for men with infections post-TRUBP. As such, more established options should be considered first. However, it does appear to penetrate well into prostate tissue [158], and may have equivalent efficacy to co-trimoxazole for prophylaxis in prostate surgery [158]. A combination of pivmecillinam with amoxicillin/clavulanate was as effective as ciprofloxacin for prophylaxis prior to TURP, and was associated with a reduction in the prevalence of ESBL-producing Enterobacteriaceae [159].
3.4.4.6 Nitroxiline
Nitroxiline is a 5-nitro-8-hydroxyquinoline drug that has been available for many years, but is not widely used outside some countries in Europe. Perhaps as a result, there is limited or no resistance to this drug in organisms like E. coli, although it has no significant activity against Pseudomonas spp. It has been effectively in the treatment of uncomplicated UTI since the 1960s and a meta-analysis of published studies including more than 10,000 patients concluded that it was non-inferior to norfloxacin or co-trimoxazole for eradication of bacteriuria and was well tolerated [160]. Whether it has a role in the treatment of MDR-infections related to TRUBP is not known, but such agents may deserve re-evaluation in this context.
3.4.4.7 Temocillin
Temocillin, a derivative of ticarcillin, has received renewed interest in recent years. It has been approved for the treatment of urinary infections in the UK and Belgium for some time, but is not widely used elsewhere. It shows stability to a range of ESBL and AmpC beta-lactamases, and even retains some activity against carbapenem-resistant KPC-producers [161], although it is inactive against Pseudomonas spp. In a study of 92 adults with mainly urinary or bloodstream infections caused by ESBL or AmpC-producers treated with temocillin, good clinical cure rates were observed [162]. It has even been used in addition to ciprofloxacin for routine prophylaxis prior to TRUBP in patients at high risk of colonisation with resistant E. coli strains [163].
3.4.5 New BLBLI drugs for MDR pathogens
In recent years there has been a renewed interest in developing novel beta-lactam/beta-lactamase inhibitor combinations [164]. Although several such compounds are in development, two new agents have recently become available for clinical use with activity against resistant Gram-negative uropathogens.
3.4.5.1 Ceftazidime/avibactam
Avibactam is a novel beta-lactamase inhibitor that has activity against ESBLs, AmpC enzymes, KPC and some OXA-type carbapenemases, although is not effective against metallo-beta-lactamases such as NDM, VIM or IMP [165]. Avibactam tends to restore the restore the activity of ceftazidime, reducing the MIC by 128-fold, usually to below clinical breakpoint against KPC [166], ESBL-producing Enterobacteriaceae [167] and AmpC-hyperproducing P. aeruginosa [168].
Several microbiological surveys have demonstrated good in vitro activity against collections of Gram negative organisms from the US, including MDR strains [169], [170], although it has limited activity against MDR Acinetobacter baumanii [168], [169]. It has recently been granted accelerated FDA approval in combination ceftazidime for the treatment of complicated UTI and abdominal infections, on the basis of Phase II trials, interim results from phase III trials and long-term safety data from ceftazidime [171]. It is interesting to note that a subset of patients with moderate baseline renal impairment experienced lower clinical cure rates, which may reflect under-dosing in patients whose renal function improved without a concomitant adjustment in the dose of ceftazidime/avibactam [171]. Although ceftazidime-avibactam is a promising agent, given the relatively limited clinical data available, it should be reserved for circumstances were no alternative effective options are available.
3.4.5.2 Ceftolozane/tazobactam
Ceftolozane is a novel cephalosporin with excellent activity against P. aeruginosa [172]. It can be inactivated by ESBLs, so is formulated with the inhibitor tazobactam. Ceftolozane/tazobactam shows good in vitro activity against a range of Gram-negative organisms, including MDR strains such as ESBL-producing Enterobacteriaceae and carbapenem-resistant P. aeruginosa [169], [173], [174]. It was granted FDA approval for the treatment of complicated UTI and intra-abdominal infections in December 2014. In a phase III randomised double-blind trial comparing ceftolozane/tazobactam with levofloxacin for the treatment of complicated UTI (the ASPECT-cUTI study), ceftolozane-tazobactam was non-inferior to levofloxacin for an endpoint of clinical cure (76.9% vs. 68.4%, 95% CI 2.3–14.6); indeed, the outcome data suggested superiority over levofloxacin, probably reflecting high rates of resistance to levofloxacin at baseline [175].
4 Management summary
| Indication | IV therapy options for sepsis/ bacteraemia if susceptible in vitro |
Oral therapy options1 if susceptible in vitro | Remarks |
|---|---|---|---|
| Enterobacteriaceae – non-MDR strains |
|
|
Use narrowest spectrum according to susceptibility results. Generally gentamicin should only be given for <48 h |
| ESBL-producing Enterobacteriaceae |
|
|
If piperacillin-tazobactam is used should be dosed maximally Generally aminoglycosides should only be given for <48 h and not used as monotherapy. Cefepime should be dosed at2 g Q8 h if normal renal function |
| AmpC-producing Enterobacteriaceae (e.g. Enterobacter cloacae/aerogenes, Citrobacter freundii, Serratia marcescens, Morganella morganii) |
|
|
|
| Pseudomonas aeruginosa |
(All +/– aminoglycoside) |
|
|
| Carbapenem-resistant/ XDR organisms |
|
|
Seek specialist advice; carbapenems may still be used if dosed to maximise exposure (e.g. extended infusions) with reference to the MIC, or used in combination |
|
1 Consider IV to oral switch once patient is afebrile, with resolved clinical signs of sepsis, tolerating oral intake, gastrointestinal absorption is not compromised and source control has been achieved; longer IV duration may be required if positive blood cultures or other complications (e.g. undrained abscess). Total duration is typically 7–14 days. |
|||
5 Further research
With decreasing effectiveness of antibiotic prophylaxis and increasing requirement for broad spectrum agents, further research is required into adjunct methods to prevent TRUBP-related complications. Currently, one randomised trial assessing targeted versus empiric antimicrobial prophylaxis is underway (ClinicalTrials.gov identifier NCT01659866), while the efficacy of PIRP is also being assessed in a randomised setting (NCT02245334; WHO ICTRP CTRI/2016/04/006843). Registered trials to assess the prevalence of fluoroquinolone resistance (NCT02140502, NCT00915213) as well as complications reported from comparisons between MRI-guided biopsy versus TRUS/transperineal biopsy, will be valuable additions to this literature.
While randomised comparisons between complications observed from TRUS and transperineal biopsy approaches are sparsely published yet desirable, it is likely that a large study population derived from multiple centres would be required to obtain statistical power. Underlying this may be attitudes and concerns from clinicians regarding randomisation, in that some who are advocates for either approach would be personally and ethically opposed to an inferior modality for their patients.
Prevention and reduction of TRUBP-related infections requires collaboration between colleagues in the fields of urology, infectious diseases and microbiology to determine the optimal prophylactic regimen, taking into account local resistance patterns and patient demographics. Future research areas in identifying the optimal solution relate to the role of targeted prophylaxis using selective rectal cultures and/or analysis of patient risk factors.
6 Conclusions
Despite heterogeneous reporting, infectious complications following prostate biopsy appear to be increasing due to fluoroquinolone resistance. Preventing TRUBP-related infections therefore requires collaboration between colleagues in the fields of urology and infectious diseases to determine the optimal prophylactic regimen, taking into account local resistance patterns and patient demographics. Nonetheless, it is clear with the decreasing effectiveness of prophylaxis and increasing use of broad spectrum agents that we require a new approach to minimising the harm of post biopsy complications. Effective preventative strategies are available, including targeted prophylaxis, extended antibiotic regimes, and alternative sampling methods, though the cost effectiveness of these strategies is yet to be elucidated. Randomised evidence is desired to establish these adjunctive tools to improve patient outcomes. In the meantime, our review supports the specific screening for risk factors predictive of post biopsy infection, to aid in the selection of patients for these preventative strategies.
7 Acknowledgement
Mathew J. Roberts is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., a Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland, Australia.
Patrick Harris is supported by an Australian Postgraduate Award from the University of Queensland.
References
[1] Banerji JS, Wolff EM, Massman JD 3rd, Odem-Davis K, Porter CR, Corman JM. Prostate Needle Biopsy Outcomes in the Era of the U.S. Preventive Services Task Force Recommendation against Prostate Specific Antigen Based Screening. J Urol. 2016 Jan;195(1):66-73. DOI: 10.1016/j.juro.2015.07.099[2] Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, Rosario DJ, Scattoni V, Lotan Y. Systematic review of complications of prostate biopsy. Eur Urol. 2013 Dec;64(6):876-92. DOI: 10.1016/j.eururo.2013.05.049
[3] Wagenlehner FM, Pilatz A, Waliszewski P, Weidner W, Johansen TE. Reducing infection rates after prostate biopsy. Nat Rev Urol. 2014 Feb;11(2):80-6. DOI: 10.1038/nrurol.2013.322
[4] Teillant A, Gandra S, Barter D, Morgan DJ, Laxminarayan R. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: a literature review and modelling study. Lancet Infect Dis. 2015 Dec;15(12):1429-37. DOI: 10.1016/S1473-3099(15)00270-4
[5] Bennett HY, Roberts MJ, Doi SA, Gardiner RA. The global burden of major infectious complications following prostate biopsy. Epidemiol Infect. 2016 Jun;144(8):1784-91. DOI: 10.1017/S0950268815002885
[6] Williamson DA, Barrett LK, Rogers BA, Freeman JT, Hadway P, Paterson DL. Infectious complications following transrectal ultrasound-guided prostate biopsy: new challenges in the era of multidrug-resistant Escherichia coli. Clin Infect Dis. 2013 Jul;57(2):267-74. DOI: 10.1093/cid/cit193
[7] Zani EL, Clark OA, Rodrigues Netto N Jr. Antibiotic prophylaxis for transrectal prostate biopsy. Cochrane Database Syst Rev. 2011 May;(5):CD006576. DOI: 10.1002/14651858.CD006576.pub2
[8] Wolf JS Jr, Bennett CJ, Dmochowski RR, Hollenbeck BK, Pearle MS, Schaeffer AJ; Urologic Surgery Antimicrobial Prophylaxis Best Practice Policy Panel. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008 Apr;179(4):1379-90. DOI: 10.1016/j.juro.2008.01.068
[9] Grabe M, Bjerklund-Johansen TE, Botto H, Wullt B, Çek M, Naber KG, Pickard RS, Tenke P, Wagenlehner F. Perioperative antibacterial prophylaxis in urology. In: Grabe M, Bjerklund-Johansen TE, Botto H, Wullt B, Çek M, Naber KG, Pickard RS, Tenke P, Wagenlehner F, editors. Guidelines on urological infections. Arnhem, The Netherlands: European Association of Urology (EAU); 2011. p. 78-94.
[10] Grabe M BR, Bjerklund-Johansen TE, Cai T, Çek M, Köves B, Naber KG, Pickard RS, Tenke P, Wagenlehner F, Wullt B. Guidelines on urological infections. European Association of Urology. 2015.
[11] Kandemir Ö, Bozlu M, Efesoy O, Güntekin O, Tek M, Akbay E. The incidence and risk factors of resistant E. coli infections after prostate biopsy under fluoroquinolone prophylaxis: a single-centre experience with 2215 patients. J Chemother. 2016 Aug;28(4):284-8. DOI: 10.1179/1973947815Y.0000000001
[12] Shandera KC, Thibault GP, Deshon GE Jr. Variability in patient preparation for prostate biopsy among American urologists. Urology. 1998 Oct;52(4):644-6. DOI: 10.1016/S0090-4295(98)00313-6
[13] Davis P, Paul E, Grummet J. Current practice of prostate biopsy in Australia and New Zealand: A survey. Urol Ann. 2015 Jul-Sep;7(3):315-9. DOI: 10.4103/0974-7796.152017
[14] Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011 Nov;186(5):1830-4. DOI: 10.1016/j.juro.2011.06.057
[15] Loeb S, van den Heuvel S, Zhu X, Bangma CH, Schröder FH, Roobol MJ. Infectious complications and hospital admissions after prostate biopsy in a European randomized trial. Eur Urol. 2012 Jun;61(6):1110-4. DOI: 10.1016/j.eururo.2011.12.058
[16] Nam RK, Saskin R, Lee Y, Liu Y, Law C, Klotz LH, Loblaw DA, Trachtenberg J, Stanimirovic A, Simor AE, Seth A, Urbach DR, Narod SA. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2010 Mar;183(3):963-8. DOI: 10.1016/j.juro.2009.11.043
[17] Carignan A, Roussy JF, Lapointe V, Valiquette L, Sabbagh R, Pépin J. Increasing risk of infectious complications after transrectal ultrasound-guided prostate biopsies: time to reassess antimicrobial prophylaxis? Eur Urol. 2012 Sep;62(3):453-9. DOI: 10.1016/j.eururo.2012.04.044
[18] Batura D, Gopal Rao G. The national burden of infections after prostate biopsy in England and Wales: a wake-up call for better prevention. J Antimicrob Chemother. 2013 Feb;68(2):247-9. DOI: 10.1093/jac/dks401
[19] Batura D, Gopal Rao G. The national burden of infections after prostate biopsy in England and Wales: a wake-up call for better prevention--authors' response. J Antimicrob Chemother. 2013 Oct;68(10):2419-20. DOI: 10.1093/jac/dkt188
[20] Roberts MJ, Parambi A, Barrett L, Hadway P, Gardiner RA, Hajkowicz KM, Yaxley J. Multifocal abscesses due to multiresistant Escherichia coli after transrectal ultrasound-guided prostate biopsy. Med J Aust. 2013 Mar;198(5):282-4. DOI: 10.5694/mja12.11719
[21] Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul;6(7):e1000097. DOI: 10.1371/journal.pmed.1000097
[22] Wagenlehner F, van Oostrum E, Tenke P, Tandogdu Z, Cek M, Grabe M, Wullt B, Pickard R, Naber KG, Pilatz A, Weidner W, Bjerklund-Johansen TE. Reply from Authors re: Riccardo Bartoletti, Tommaso Cai. Prostate Biopsies Should Be Performed According to a Standard of Care. Eur Urol 2013;63:528-9. Eur Urol. 2013 Mar;63(3):529-30. DOI: 10.1016/j.eururo.2012.07.037
[23] Gopal Rao G, Batura D. Emergency hospital admissions attributable to infective complications of prostate biopsy despite appropriate prophylaxis: need for additional infection prevention strategies? Int Urol Nephrol. 2014 Feb;46(2):309-15. DOI: 10.1007/s11255-013-0529-5
[24] Womble PR, Dixon MW, Linsell SM, Ye Z, Montie JE, Lane BR, Miller DC, Burks FN; Michigan Urological Surgery Improvement Collaborative. Infection related hospitalizations after prostate biopsy in a statewide quality improvement collaborative. J Urol. 2014 Jun;191(6):1787-92. DOI: 10.1016/j.juro.2013.12.026
[25] Wagenlehner FM, van Oostrum E, Tenke P, Tandogdu Z, Çek M, Grabe M, Wullt B, Pickard R, Naber KG, Pilatz A, Weidner W, Bjerklund-Johansen TE; GPIU investigators. Infective complications after prostate biopsy: outcome of the Global Prevalence Study of Infections in Urology (GPIU) 2010 and 2011, a prospective multinational multicentre prostate biopsy study. Eur Urol. 2013 Mar;63(3):521-7. DOI: 10.1016/j.eururo.2012.06.003
[26] Carmignani L, Picozzi S, Spinelli M, Di Pierro S, Mombelli G, Negri E, Tejada M, Gaia P, Costa E, Maggioni A. Bacterial sepsis following prostatic biopsy. Int Urol Nephrol. 2012 Aug;44(4):1055-63. DOI: 10.1007/s11255-012-0145-9
[27] Liss MA, Chang A, Santos R, Nakama-Peeples A, Peterson EM, Osann K, Billimek J, Szabo RJ, Dash A. Prevalence and significance of fluoroquinolone resistant Escherichia coli in patients undergoing transrectal ultrasound guided prostate needle biopsy. J Urol. 2011 Apr;185(4):1283-8. DOI: 10.1016/j.juro.2010.11.088
[28] Williamson DA, Roberts SA, Paterson DL, Sidjabat H, Silvey A, Masters J, Rice M, Freeman JT. Escherichia coli bloodstream infection after transrectal ultrasound-guided prostate biopsy: implications of fluoroquinolone-resistant sequence type 131 as a major causative pathogen. Clin Infect Dis. 2012 May;54(10):1406-12. DOI: 10.1093/cid/cis194
[29] Zaytoun OM, Vargo EH, Rajan R, Berglund R, Gordon S, Jones JS. Emergence of fluoroquinolone-resistant Escherichia coli as cause of postprostate biopsy infection: implications for prophylaxis and treatment. Urology. 2011 May;77(5):1035-41. DOI: 10.1016/j.urology.2010.12.067
[30] Campeggi A, Ouzaid I, Xylinas E, Lesprit P, Hoznek A, Vordos D, Abbou CC, Salomon L, de la Taille A. Acute bacterial prostatitis after transrectal ultrasound-guided prostate biopsy: epidemiological, bacteria and treatment patterns from a 4-year prospective study. Int J Urol. 2014 Feb;21(2):152-5. DOI: 10.1111/iju.12207
[31] Lundström KJ, Drevin L, Carlsson S, Garmo H, Loeb S, Stattin P, Bill-Axelson A. Nationwide population based study of infections after transrectal ultrasound guided prostate biopsy. J Urol. 2014 Oct;192(4):1116-22. DOI: 10.1016/j.juro.2014.04.098
[32] Zowawi HM, Harris PN, Roberts MJ, Tambyah PA, Schembri MA, Pezzani MD, Williamson DA, Paterson DL. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol. 2015 Oct;12(10):570-84. DOI: 10.1038/nrurol.2015.199
[33] Anderson E, Leahy O, Cheng AC, Grummet J. Risk factors for infection following prostate biopsy - a case control study. BMC Infect Dis. 2015 Dec;15:580. DOI: 10.1186/s12879-015-1328-7
[34] Liss MA, Johnson JR, Porter SB, Johnston B, Clabots C, Gillis K, Nseyo U, Holden M, Sakamoto K, Fierer J. Clinical and microbiological determinants of infection after transrectal prostate biopsy. Clin Infect Dis. 2015 Apr;60(7):979-87. DOI: 10.1093/cid/ciu1129
[35] Roberts MJ, Williamson DA, Hadway P, Doi SA, Gardiner RA, Paterson DL. Baseline prevalence of antimicrobial resistance and subsequent infection following prostate biopsy using empirical or altered prophylaxis: A bias-adjusted meta-analysis. Int J Antimicrob Agents. 2014 Apr;43(4):301-9. DOI: 10.1016/j.ijantimicag.2014.01.008
[36] Patel U, Dasgupta P, Amoroso P, Challacombe B, Pilcher J, Kirby R. Infection after transrectal ultrasonography-guided prostate biopsy: increased relative risks after recent international travel or antibiotic use. BJU Int. 2012 Jun;109(12):1781-5. DOI: 10.1111/j.1464-410X.2011.10561.x
[37] Rogers BA, Aminzadeh Z, Hayashi Y, Paterson DL. Country-to-country transfer of patients and the risk of multi-resistant bacterial infection. Clin Infect Dis. 2011 Jul;53(1):49-56. DOI: 10.1093/cid/cir273
[38] Williamson DA, Masters J, Freeman J, Roberts S. Travel-associated extended-spectrum β-lactamase-producing Escherichia coli bloodstream infection following transrectal ultrasound-guided prostate biopsy. BJU Int. 2012 Apr;109(7):E21-2. DOI: 10.1111/j.1464-410X.2012.11001.x
[39] Lee JW, Park SC, Kim MK, Cheon MW, Kim GY, Cho JH. Prevalence of antimicrobial resistance in normal rectal flora of patients undergoing transrectal ultrasonography-guided prostate biopsy in Korea. Int J Urol. 2014 Aug;21(8):811-4. DOI: 10.1111/iju.12454
[40] Siriboon S, Tiengrim S, Taweemongkongsup T, Thamlikitkul V, Chayakulkeeree M. Prevalence of antibiotic resistance in fecal flora of patients undergoing transrectal ultrasound-guided prostate biopsy in Thailand. Urol Int. 2012;88(2):187-93. DOI: 10.1159/000335506
[41] Liss MA, Taylor SA, Batura D, Steensels D, Chayakulkeeree M, Soenens C, Rao GG, Dash A, Park S, Patel N, Woo J, McDonald M, Nseyo U, Banapour P, Unterberg S, Ahlering TE, Van Poppel H, Sakamoto K, Fierer J, Black PC. Fluoroquinolone resistant rectal colonization predicts risk of infectious complications after transrectal prostate biopsy. J Urol. 2014 Dec;192(6):1673-8. DOI: 10.1016/j.juro.2014.06.005
[42] Cohen JE, Landis P, Trock BJ, Patel HD, Ball MW, Auwaerter PG, Schaeffer E, Carter HB. Fluoroquinolone resistance in the rectal carriage of men in an active surveillance cohort: longitudinal analysis. J Urol. 2015 Feb;193(2):552-6. DOI: 10.1016/j.juro.2014.08.008
[43] Qi C, Malczynski M, Schaeffer AJ, Barajas G, Nadler RB, Scheetz MH, Zembower TR. Characterization of ciprofloxacin resistant Escherichia coli isolates among men undergoing evaluation for transrectal ultrasound guided prostate biopsy. J Urol. 2013 Dec;190(6):2026-32. DOI: 10.1016/j.juro.2013.05.059
[44] Roberts MJ, Doi SA. Prostate biopsy, targeted prophylaxis and infectious complications: a critique of methods used. BJU Int. 2016 May;117(5):719-21. DOI: 10.1111/bju.13466
[45] Bruyère F, Malavaud S, Bertrand P, Decock A, Cariou G, Doublet JD, Bernard L, Bugel H, Conquy S, Sotto A, Boiteux JP, Pogu B, Rebillard X, Mongiat-Artus P, Coloby P. Prosbiotate: a multicenter, prospective analysis of infectious complications after prostate biopsy. J Urol. 2015 Jan;193(1):145-50. DOI: 10.1016/j.juro.2014.07.086
[46] Steensels D, Slabbaert K, De Wever L, Vermeersch P, Van Poppel H, Verhaegen J. Fluoroquinolone-resistant E. coli in intestinal flora of patients undergoing transrectal ultrasound-guided prostate biopsy--should we reassess our practices for antibiotic prophylaxis? Clin Microbiol Infect. 2012 Jun;18(6):575-81. DOI: 10.1111/j.1469-0691.2011.03638.x
[47] Taylor S, Margolick J, Abughosh Z, Goldenberg SL, Lange D, Bowie WR, Bell R, Roscoe D, Machan L, Black P. Ciprofloxacin resistance in the faecal carriage of patients undergoing transrectal ultrasound guided prostate biopsy. BJU Int. 2013 May;111(6):946-53. DOI: 10.1111/j.1464-410X.2012.11637.x
[48] Anderson E, Leahy O, Cheng AC, Grummet J. Risk factors for infection following prostate biopsy - a case control study. BMC Infect Dis. 2015 Dec;15:580. DOI: 10.1186/s12879-015-1328-7
[49] Horcajada JP, Busto M, Grau S, Sorlí L, Terradas R, Salvadó M, Lorente JA, González A, Knobel H. High prevalence of extended-spectrum beta-lactamase-producing enterobacteriaceae in bacteremia after transrectal ultrasound-guided prostate biopsy: a need for changing preventive protocol. Urology. 2009 Dec;74(6):1195-9. DOI: 10.1016/j.urology.2009.06.061
[50] Wagenlehner F, Stöwer-Hoffmann J, Schneider-Brachert W, Naber KG, Lehn N. Influence of a prophylactic single dose of ciprofloxacin on the level of resistance of Escherichia coli to fluoroquinolones in urology. Int J Antimicrob Agents. 2000 Aug;15(3):207-11. DOI: 10.1016/S0924-8579(00)00182-5
[51] Akduman B, Akduman D, Tokgöz H, Erol B, Türker T, Ayoğlu F, Mungan NA. Long-term fluoroquinolone use before the prostate biopsy may increase the risk of sepsis caused by resistant microorganisms. Urology. 2011 Aug;78(2):250-5. DOI: 10.1016/j.urology.2011.02.065
[52] Arslan H, Azap OK, Ergönül O, Timurkaynak F; Urinary Tract Infection Study Group. Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J Antimicrob Chemother. 2005 Nov;56(5):914-8. DOI: 10.1093/jac/dki344
[53] Dumford D 3rd, Suwantarat N, Bhasker V, Kundrapu S, Zabarsky TF, Drawz P, Zhu H, Donskey CJ. Outbreak of fluoroquinolone-resistant Escherichia coli infections after transrectal ultrasound-guided biopsy of the prostate. Infect Control Hosp Epidemiol. 2013 Mar;34(3):269-73. DOI: 10.1086/669512
[54] Carlson WH, Bell DG, Lawen JG, Rendon RA. Multi-drug resistant E.coli urosepsis in physicians following transrectal ultrasound guided prostate biopsies--three cases including one death. Can J Urol. 2010 Apr;17(2):5135-7.
[55] Kamdar C, Mooppan UM, Gulmi FA, Kim H. Multi-drug-resistant bacteremia after transrectal ultrasound guided prostate biopsies in hospital employees and their relatives. Urology. 2008 Jul;72(1):34-6. DOI: 10.1016/j.urology.2008.01.065
[56] Losco G, Studd R, Blackmore T. Ertapenem prophylaxis reduces sepsis after transrectal biopsy of the prostate. BJU Int. 2014 Mar;113 Suppl 2:69-72. DOI: 10.1111/bju.12590
[57] Lindstedt S, Lindström U, Ljunggren E, Wullt B, Grabe M. Single-dose antibiotic prophylaxis in core prostate biopsy: Impact of timing and identification of risk factors. Eur Urol. 2006 Oct;50(4):832-7. DOI: 10.1016/j.eururo.2006.05.003
[58] Wagenlehner FM, van Oostrum E, Tenke P, Tandogdu Z, Çek M, Grabe M, Wullt B, Pickard R, Naber KG, Pilatz A, Weidner W, Bjerklund-Johansen TE; GPIU investigators. Infective complications after prostate biopsy: outcome of the Global Prevalence Study of Infections in Urology (GPIU) 2010 and 2011, a prospective multinational multicentre prostate biopsy study. Eur Urol. 2013 Mar;63(3):521-7. DOI: 10.1016/j.eururo.2012.06.003
[59] Simsir A, Kismali E, Mammadov R, Gunaydin G, Cal C. Is it possible to predict sepsis, the most serious complication in prostate biopsy? Urol Int. 2010;84(4):395-9. DOI: 10.1159/000296290
[60] Anastasiadis E, van der Meulen J, Emberton M. Hospital admissions after transrectal ultrasound-guided biopsy of the prostate in men diagnosed with prostate cancer: a database analysis in England. Int J Urol. 2015 Feb;22(2):181-6. DOI: 10.1111/iju.12634
[61] Luong B, Danforth T, Visnjevac O, Suraf M, Duff M, Chevli KK. Reduction in hospital admissions with the addition of prophylactic intramuscular ceftriaxone before transrectal ultrasonography-guided prostate biopsies. Urology. 2015 Mar;85(3):511-6. DOI: 10.1016/j.urology.2014.10.047
[62] Sahin C, Eryildirim B, Cetinel AC, Faydaci G, Narter F, Goktas C, Sarica K. Does metabolic syndrome increase the risk of infective complications after prostate biopsy? A critical evaluation. Int Urol Nephrol. 2015 Mar;47(3):423-9. DOI: 10.1007/s11255-014-0904-x
[63] Tsu JH, Ma WK, Chan WK, Lam BH, To KC, To WK, Ng TK, Liu PL, Cheung FK, Yiu MK. Prevalence and predictive factors of harboring fluoroquinolone-resistant and extended-spectrum β-lactamase-producing rectal flora in Hong Kong Chinese men undergoing transrectal ultrasound-guided prostate biopsy. Urology. 2015 Jan;85(1):15-21. DOI: 10.1016/j.urology.2014.07.078
[64] Suwantarat N, Dumford DM 3rd, Ponce-Terashima R, Kundrapu S, Zabarsky TF, Zhu H, Donskey CJ. Modification of antimicrobial prophylaxis based on rectal culture results to prevent fluoroquinolone-resistant Escherichia coli infections after prostate biopsy. Infect Control Hosp Epidemiol. 2013 Sep;34(9):973-6. DOI: 10.1086/671734
[65] Hossack T, Patel MI, Huo A, Brenner P, Yuen C, Spernat D, Mathews J, Haynes AM, Sutherland R, del Prado W, Stricker P. Location and pathological characteristics of cancers in radical prostatectomy specimens identified by transperineal biopsy compared to transrectal biopsy. J Urol. 2012 Sep;188(3):781-5. DOI: 10.1016/j.juro.2012.05.006
[66] Ong WL, Weerakoon M, Huang S, Paul E, Lawrentschuk N, Frydenberg M, Moon D, Murphy D, Grummet J. Transperineal biopsy prostate cancer detection in first biopsy and repeat biopsy after negative transrectal ultrasound-guided biopsy: the Victorian Transperineal Biopsy Collaboration experience. BJU Int. 2015 Oct;116(4):568-76. DOI: 10.1111/bju.13031
[67] Dundee PE, Grummet JP, Murphy DG. Transperineal prostate biopsy: template-guided or freehand? BJU Int. 2015 May;115(5):681-3. DOI: 10.1111/bju.12860
[68] Grummet JP, Weerakoon M, Huang S, Lawrentschuk N, Frydenberg M, Moon DA, O'Reilly M, Murphy D. Sepsis and 'superbugs': should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int. 2014 Sep;114(3):384-8. DOI: 10.1111/bju.12536
[69] Namekawa T, Fukasawa S, Komaru A, Kobayashi M, Imamura Y, Ohzeki T, Takagi K, Sato Y, Akakura K, Ichikawa T, Ueda T. Prospective evaluation of the safety of transrectal ultrasound-guided transperineal prostate biopsy based on adverse events. Int J Clin Oncol. 2015 Dec;20(6):1185-91. DOI: 10.1007/s10147-015-0831-6
[70] Mai Z, Yan W, Zhou Y, Zhou Z, Chen J, Xiao Y, Liang Z, Ji Z, Li H. Transperineal template-guided prostate biopsy: 10 years of experience. BJU Int. 2016 Mar;117(3):424-9. DOI: 10.1111/bju.13024
[71] Pepe P, Aragona F. Morbidity after transperineal prostate biopsy in 3000 patients undergoing 12 vs 18 vs more than 24 needle cores. Urology. 2013 Jun;81(6):1142-6. DOI: 10.1016/j.urology.2013.02.019
[72] Eichler K, Hempel S, Wilby J, Myers L, Bachmann LM, Kleijnen J. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol. 2006 May;175(5):1605-12. DOI: 10.1016/S0022-5347(05)00957-2
[73] Berger AP, Gozzi C, Steiner H, Frauscher F, Varkarakis J, Rogatsch H, Bartsch G, Horninger W. Complication rate of transrectal ultrasound guided prostate biopsy: a comparison among 3 protocols with 6, 10 and 15 cores. J Urol. 2004 Apr;171(4):1478-80; discussion 1480-1. DOI: 10.1097/01.ju.0000116449.01186.f7
[74] Scattoni V, Maccagnano C, Zanni G, Angiolilli D, Raber M, Roscigno M, Rigatti P, Montorsi F. Is extended and saturation biopsy necessary? Int J Urol. 2010 May;17(5):432-47. DOI: 10.1111/j.1442-2042.2010.02479.x
[75] Inal GH, Oztekin VC, Uğurlu O, Kosan M, Akdemir O, Cetinkaya M. Sixteen gauge needles improve specimen quality but not cancer detection rate in transrectal ultrasound-guided 10-core prostate biopsies. Prostate Cancer Prostatic Dis. 2008;11(3):270-3. DOI: 10.1038/pcan.2008.34
[76] Ukimura O, Coleman JA, de la Taille A, Emberton M, Epstein JI, Freedland SJ, Giannarini G, Kibel AS, Montironi R, Ploussard G, Roobol MJ, Scattoni V, Jones JS. Contemporary role of systematic prostate biopsies: indications, techniques, and implications for patient care. Eur Urol. 2013 Feb;63(2):214-30. DOI: 10.1016/j.eururo.2012.09.033
[77] Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Is repeat prostate biopsy associated with a greater risk of hospitalization? Data from SEER-Medicare. J Urol. 2013 Mar;189(3):867-70. DOI: 10.1016/j.juro.2012.10.005
[78] Ehdaie B, Vertosick E, Spaliviero M, Giallo-Uvino A, Taur Y, O'Sullivan M, Livingston J, Sogani P, Eastham J, Scardino P, Touijer K. The impact of repeat biopsies on infectious complications in men with prostate cancer on active surveillance. J Urol. 2014 Mar;191(3):660-4. DOI: 10.1016/j.juro.2013.08.088
[79] Liss MA, Nakamura KK, Meuleners R, Kolla SB, Dash A, Peterson EM. Screening rectal culture to identify fluoroquinolone-resistant organisms before transrectal prostate biopsy: do the culture results between office visit and biopsy correlate? Urology. 2013 Jul;82(1):67-71. DOI: 10.1016/j.urology.2013.02.068
[80] Liss M, Nakamura K, Peterson E. Targeted prophylaxis prior to transrectal prostate biopsy: A comparison of broth enrichment to direct plating for the evaluation of rectal cultures. J Urol. 2012;187:e439. DOI: 10.1016/j.juro.2012.02.118
[81] Taylor AK, Zembower TR, Nadler RB, Scheetz MH, Cashy JP, Bowen D, Murphy AB, Dielubanza E, Schaeffer AJ. Targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound guided prostate biopsy is associated with reduced incidence of postoperative infectious complications and cost of care. J Urol. 2012 Apr;187(4):1275-9. DOI: 10.1016/j.juro.2011.11.115
[82] Duplessis CA, Bavaro M, Simons MP, Marguet C, Santomauro M, Auge B, Collard DA, Fierer J, Lesperance J. Rectal cultures before transrectal ultrasound-guided prostate biopsy reduce post-prostatic biopsy infection rates. Urology. 2012 Mar;79(3):556-61. DOI: 10.1016/j.urology.2011.09.057
[83] Dai J, Leone A, Mermel L, Hwang K, Pareek G, Schiff S, Golijanin D, Renzulli JF 2nd. Rectal swab culture-directed antimicrobial prophylaxis for prostate biopsy and risk of postprocedure infection: a cohort study. Urology. 2015 Jan;85(1):8-14. DOI: 10.1016/j.urology.2014.09.035
[84] Farrell J, Salter C, Cullen J, Mordkin R, Joel A. Culture Directed Antibiotic Prophylaxis Reduces Post-Prostate Biopsy Infectious Complications in the Community: A "How-to" for Urologists in the Trenches. Urology Practice. 2015;2(4):166-171. DOI: 10.1016/j.urpr.2014.10.012
[85] Wright WL, Larking P, Lovell-Smith CJ. Concentrations of trimethoprim and sulphamethoxazole in the human prostate gland after intramuscular injection. Br J Urol. 1982 Oct;54(5):550-1. DOI: 10.1111/j.1464-410X.1982.tb13588.x
[86] Gardiner BJ, Mahony AA, Ellis AG, Lawrentschuk N, Bolton DM, Zeglinski PT, Frauman AG, Grayson ML. Is fosfomycin a potential treatment alternative for multidrug-resistant gram-negative prostatitis? Clin Infect Dis. 2014 Feb;58(4):e101-5. DOI: 10.1093/cid/cit704
[87] Davis M, Sofer M, Kim SS, Soloway MS. The procedure of transrectal ultrasound guided biopsy of the prostate: a survey of patient preparation and biopsy technique. J Urol. 2002 Feb;167(2 Pt 1):566-70. DOI: 10.1016/S0022-5347(01)69087-6
[88] Pattana-arun J, Wolff BG. Benefits of povidone-iodine solution in colorectal operations: science or legend. Dis Colon Rectum. 2008 Jun;51(6):966-71. DOI: 10.1007/s10350-008-9213-8
[89] Pu C, Bai Y, Yuan H, Li J, Tang Y, Wang J, Wei Q, Han P. Reducing the risk of infection for transrectal prostate biopsy with povidone-iodine: a systematic review and meta-analysis. Int Urol Nephrol. 2014 Sep;46(9):1691-8. DOI: 10.1007/s11255-014-0713-2
[90] Gyorfi JR, Otteni C, Brown K, Patel A, Lehman K, Phillips BE, Dewan K, Kirimanjeswara G, Raman JD. Peri-procedural povidone-iodine rectal preparation reduces microorganism counts and infectious complications following ultrasound-guided needle biopsy of the prostate. World J Urol. 2014 Aug;32(4):905-9. DOI: 10.1007/s00345-014-1291-8
[91] Abughosh Z, Margolick J, Goldenberg SL, Taylor SA, Afshar K, Bell R, Lange D, Bowie WR, Roscoe D, Machan L, Black PC. A prospective randomized trial of povidone-iodine prophylactic cleansing of the rectum before transrectal ultrasound guided prostate biopsy. J Urol. 2013 Apr;189(4):1326-31. DOI: 10.1016/j.juro.2012.09.121
[92] Park DS, Hwang JH, Choi DK, Gong IH, Hong YK, Park S, Oh JJ. Control of infective complications of transrectal prostate biopsy. Surg Infect (Larchmt). 2014 Aug;15(4):431-6. DOI: 10.1089/sur.2013.138
[93] Goh YS, Law ZW, Tiong HY. Re: A prospective randomized trial of povidone-iodine prophylactic cleansing of the rectum before transrectal ultrasound guided prostate biopsy: Z. AbuGhosh, J. Margolick, S. L. Goldenberg, S. A. Taylor, K. Afshar, R. Bell, D. Lange, W. R. Bowie, D. Roscoe, L. Machan and P. C. Black J Urol 2013; 189: 1326-1331. J Urol. 2013 Dec;190(6):2309-10. DOI: 10.1016/j.juro.2013.07.010
[94] Lee G, Attar K, Laniado M, Karim O. Trans-rectal ultrasound guided biopsy of the prostate: nationwide diversity in practice and training in the United Kingdom. Int Urol Nephrol. 2007;39(1):185-8. DOI: 10.1007/s11255-006-6654-7
[95] Lindert KA, Kabalin JN, Terris MK. Bacteremia and bacteriuria after transrectal ultrasound guided prostate biopsy. J Urol. 2000 Jul;164(1):76-80. DOI: 10.1016/S0022-5347(05)67453-8
[96] Zani EL, Clark OA, Rodrigues Netto N Jr. Antibiotic prophylaxis for transrectal prostate biopsy. Cochrane Database Syst Rev. 2011 May 11;(5):CD006576. DOI: 10.1002/14651858.CD006576.pub2
[97] Jeon SS, Woo SH, Hyun JH, Choi HY, Chai SE. Bisacodyl rectal preparation can decrease infectious complications of transrectal ultrasound-guided prostate biopsy. Urology. 2003 Sep;62(3):461-6. DOI: 10.1016/S0090-4295(03)00470-9
[98] Carey JM, Korman HJ. Transrectal ultrasound guided biopsy of the prostate. Do enemas decrease clinically significant complications? J Urol. 2001 Jul;166(1):82-5. DOI: 10.1016/S0022-5347(05)66082-X
[99] De Nunzio C, Lombardo R, Presicce F, Bellangino M, Finazzi Agro E, Gambrosier MB, Trucchi A, Petta S, Tubaro A. Transrectal-ultrasound prostatic biopsy preparation: rectal enema vs. mechanical bowel preparation. Cent European J Urol. 2015;68(2):223-8. DOI: 10.5173/ceju.2015.608
[100] Issa MM, Al-Qassab UA, Hall J, Ritenour CW, Petros JA, Sullivan JW. Formalin disinfection of biopsy needle minimizes the risk of sepsis following prostate biopsy. J Urol. 2013 Nov;190(5):1769-75. DOI: 10.1016/j.juro.2013.04.134
[101] Borkowetz A, Platzek I, Toma M, Laniado M, Baretton G, Froehner M, Koch R, Wirth M, Zastrow S. Comparison of systematic transrectal biopsy to transperineal magnetic resonance imaging/ultrasound-fusion biopsy for the diagnosis of prostate cancer. BJU Int. 2015 Dec;116(6):873-9. DOI: 10.1111/bju.13023
[102] Pokorny MR, de Rooij M, Duncan E, Schröder FH, Parkinson R, Barentsz JO, Thompson LC. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014 Jul;66(1):22-9. DOI: 10.1016/j.eururo.2014.03.002
[103] Radtke JP, Teber D, Hohenfellner M, Hadaschik BA. The current and future role of magnetic resonance imaging in prostate cancer detection and management. Transl Androl Urol. 2015 Jun;4(3):326-41. DOI: 10.3978/j.issn.2223-4683.2015.06.05
[104] Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother. 2007 Nov;60(5):913-20. DOI: 10.1093/jac/dkm318
[105] Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B, Citton R, D'Inzeo T, Fadda G, Cauda R, Spanu T. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother. 2007 Jun;51(6):1987-94. DOI: 10.1128/AAC.01509-06
[106] Trecarichi EM, Cauda R, Tumbarello M. Detecting risk and predicting patient mortality in patients with extended-spectrum β-lactamase-producing Enterobacteriaceae bloodstream infections. Future Microbiol. 2012 Oct;7(10):1173-89. DOI: 10.2217/fmb.12.100
[107] Rogers BA, Ingram PR, Runnegar N, Pitman MC, Freeman JT, Athan E, Havers SM, Sidjabat HE, Jones M, Gunning E, De Almeida M, Styles K, Paterson DL; Australasian Society for Infectious Diseases Clinical Research Network. Community-onset Escherichia coli infection resistant to expanded-spectrum cephalosporins in low-prevalence countries. Antimicrob Agents Chemother. 2014;58(4):2126-34. DOI: 10.1128/AAC.02052-13
[108] European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2014. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: ECDC; 2015. Available from: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/antimicrobial-resistance-europe-2014.pdf
[109] World Health Organisation. Antimicrobial Resistance Global Report on surveillance. Geneva: World Health Organisation; 2014. Available from: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1
[110] Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012 Mar;18(3):268-81. DOI: 10.1111/j.1469-0691.2011.03570.x
[111] Zowawi HM, Forde BM, Alfaresi M, Alzarouni A, Farahat Y, Chong TM, Yin WF, Chan KG, Li J, Schembri MA, Beatson SA, Paterson DL. Stepwise evolution of pandrug-resistance in Klebsiella pneumoniae. Sci Rep. 2015 Oct;5:15082. DOI: 10.1038/srep15082
[112] Williamson DA, Freeman JT, Roberts SA, Heffernan H, Dyet K, Paterson DL, Rogers BA, Sidjabat HE, Masters J. Rectal colonization with New Delhi metallo-β-lactamase-1-producing Escherichia coli prior to transrectal ultrasound (TRUS)-guided prostate biopsy. J Antimicrob Chemother. 2013 Dec;68(12):2957-9. DOI: 10.1093/jac/dkt266
[113] Tumbarello M, Trecarichi EM, Bassetti M, De Rosa FG, Spanu T, Di Meco E, Losito AR, Parisini A, Pagani N, Cauda R. Identifying patients harboring extended-spectrum-beta-lactamase-producing Enterobacteriaceae on hospital admission: derivation and validation of a scoring system. Antimicrob Agents Chemother. 2011 Jul;55(7):3485-90. DOI: 10.1128/AAC.00009-11
[114] Johnson SW, Anderson DJ, May DB, Drew RH. Utility of a clinical risk factor scoring model in predicting infection with extended-spectrum β-lactamase-producing enterobacteriaceae on hospital admission. Infect Control Hosp Epidemiol. 2013 Apr;34(4):385-92. DOI: 10.1086/669858
[115] Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013 Feb;41(2):580-637. DOI: 10.1097/CCM.0b013e31827e83af
[116] Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016 Feb;315(8):801-10. DOI: 10.1001/jama.2016.0287
[117] Sanders A, Buchan N. Infection-related hospital admissions after transrectal biopsy of the prostate. ANZ J Surg. 2013 Apr;83(4):246-8. DOI: 10.1111/ans.12073
[118] Therapeutic Guidelines Limited. Australian Therapeutic Guidelines: Antibiotic. 15th Ed. 2014. Available from: https://www.clinicalguidelines.gov.au/portal/2406/therapeutic-guidelines-antibiotic-version-15
[119] Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis. 2012 Jun;54(12):1699-709. DOI: 10.1093/cid/cis270
[120] Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005 Oct;18(4):657-86. DOI: 10.1128/CMR.18.4.657-686.2005
[121] Pitout JD. Infections with extended-spectrum beta-lactamase-producing enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs. 2010 Feb;70(3):313-33. DOI: 10.2165/11533040-000000000-00000
[122] Nicolau DP. Carbapenems: a potent class of antibiotics. Expert Opin Pharmacother. 2008 Jan;9(1):23-37. DOI: 10.1517/14656566.9.1.23
[123] Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009 Jan;22(1):161-82, Table of Contents. DOI: 10.1128/CMR.00036-08
[124] Du B, Long Y, Liu H, Chen D, Liu D, Xu Y, Xie X. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae bloodstream infection: risk factors and clinical outcome. Intensive Care Med. 2002 Dec;28(12):1718-23. DOI: 10.1007/s00134-002-1521-1
[125] Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, Bonomo RA, Rice LB, Wagener MM, McCormack JG, Yu VL. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis. 2004 Jul;39(1):31-7. DOI: 10.1086/420816
[126] Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, Bonomo RA, Rice LB, Wagener MM, McCormack JG, Yu VL. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med. 2004 Jan;140(1):26-32.
[127] Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2012 Dec;67(12):2793-803. DOI: 10.1093/jac/dks301
[128] Kang CI, Cha MK, Kim SH, Wi YM, Chung DR, Peck KR, Lee NY, Song JH. Extended-spectrum cephalosporins and the inoculum effect in tests with CTX-M-type extended-spectrum β-lactamase-producing Escherichia coli: potential clinical implications of the revised CLSI interpretive criteria. Int J Antimicrob Agents. 2014 May;43(5):456-9. DOI: 10.1016/j.ijantimicag.2014.01.030
[129] Lee NY, Lee CC, Huang WH, Tsui KC, Hsueh PR, Ko WC. Cefepime therapy for monomicrobial bacteremia caused by cefepime-susceptible extended-spectrum beta-lactamase-producing Enterobacteriaceae: MIC matters. Clin Infect Dis. 2013 Feb;56(4):488-95. DOI: 10.1093/cid/cis916
[130] Nguyen HM, Shier KL, Graber CJ. Determining a clinical framework for use of cefepime and β-lactam/β-lactamase inhibitors in the treatment of infections caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother. 2014 Apr;69(4):871-80. DOI: 10.1093/jac/dkt450
[131] Tamma PD, Girdwood SC, Gopaul R, Tekle T, Roberts AA, Harris AD, Cosgrove SE, Carroll KC. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis. 2013 Sep;57(6):781-8. DOI: 10.1093/cid/cit395
[132] Hilty M, Sendi P, Seiffert SN, Droz S, Perreten V, Hujer AM, Bonomo RA, Mühlemann K, Endimiani A. Characterisation and clinical features of Enterobacter cloacae bloodstream infections occurring at a tertiary care university hospital in Switzerland: is cefepime adequate therapy? Int J Antimicrob Agents. 2013 Mar;41(3):236-49. DOI: 10.1016/j.ijantimicag.2012.10.022
[133] Harris PN, Wei JY, Shen AW, Abdile AA, Paynter S, Huxley RR, Pandeya N, Doi Y, Huh K, O'Neal CS, Talbot TR, Paterson DL. Carbapenems versus alternative antibiotics for the treatment of bloodstream infections caused by Enterobacter, Citrobacter or Serratia species: a systematic review with meta-analysis. J Antimicrob Chemother. 2016 Feb;71(2):296-306. DOI: 10.1093/jac/dkv346
[134] Lee NY, Lee CC, Li CW, Li MC, Chen PL, Chang CM, Ko WC. Cefepime Therapy for Monomicrobial Enterobacter cloacae Bacteremia: Unfavorable Outcomes in Patients Infected by Cefepime-Susceptible Dose-Dependent Isolates. Antimicrob Agents Chemother. 2015 Dec;59(12):7558-63. DOI: 10.1128/AAC.01477-15
[135] Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis. 2007 May;7(5):338-48. DOI: 10.1016/S1473-3099(07)70109-3
[136] Kim PW, Wu YT, Cooper C, Rochester G, Valappil T, Wang Y, Kornegay C, Nambiar S. Meta-analysis of a possible signal of increased mortality associated with cefepime use. Clin Infect Dis. 2010 Aug;51(4):381-9. DOI: 10.1086/655131
[137] Roos JF, Bulitta J, Lipman J, Kirkpatrick CM. Pharmacokinetic-pharmacodynamic rationale for cefepime dosing regimens in intensive care units. J Antimicrob Chemother. 2006 Nov;58(5):987-93. DOI: 10.1093/jac/dkl349
[138] Harris PN, Tambyah PA, Paterson DL. β-lactam and β-lactamase inhibitor combinations in the treatment of extended-spectrum β-lactamase producing Enterobacteriaceae: time for a reappraisal in the era of few antibiotic options? Lancet Infect Dis. 2015 Apr;15(4):475-85. DOI: 10.1016/S1473-3099(14)70950-8
[139] Thomson KS, Moland ES. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2001 Dec;45(12):3548-54. DOI: 10.1128/AAC.45.12.3548-3554.2001
[140] Perez F, Bonomo RA. Can we really use ß-lactam/ß-lactam inhibitor combinations for the treatment of infections caused by extended-spectrum ß-lactamase-producing bacteria? Clin Infect Dis. 2012 Jan 15;54(2):175-7. DOI: 10.1093/cid/cir793
[141] Harada Y, Morinaga Y, Kaku N, Nakamura S, Uno N, Hasegawa H, Izumikawa K, Kohno S, Yanagihara K. In vitro and in vivo activities of piperacillin-tazobactam and meropenem at different inoculum sizes of ESBL-producing Klebsiella pneumoniae. Clin Microbiol Infect. 2014 Nov;20(11):O831-9. DOI: 10.1111/1469-0691.12677
[142] Rodríguez-Baño J, Navarro MD, Retamar P, Picón E, Pascual Á; Extended-Spectrum Beta-Lactamases–Red Española de Investigación en Patología Infecciosa/Grupo de Estudio de Infección Hospitalaria Group. β-Lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012 Jan;54(2):167-74. DOI: 10.1093/cid/cir790
[143] Harris PN, Yin M, Jureen R, Chew J, Ali J, Paynter S, Paterson DL, Tambyah PA. Comparable outcomes for β-lactam/β-lactamase inhibitor combinations and carbapenems in definitive treatment of bloodstream infections caused by cefotaxime-resistant Escherichia coli or Klebsiella pneumoniae. Antimicrob Resist Infect Control. 2015;4:14. DOI: 10.1186/s13756-015-0055-6
[144] Ng TM, Khong WX, Harris PN, De PP, Chow A, Tambyah PA, Lye DC. Empiric Piperacillin-Tazobactam versus Carbapenems in the Treatment of Bacteraemia Due to Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae. PLoS ONE. 2016;11(4):e0153696. DOI: 10.1371/journal.pone.0153696
[145] Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, Avdic E, Cosgrove SE; Antibacterial Resistance Leadership Group. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis. 2015 May 1;60(9):1319-25. DOI: 10.1093/cid/civ003
[146] Gutiérrez-Gutiérrez B, Pérez-Galera S, Salamanca E, de Cueto M, Calbo E, Almirante B, Viale P, Oliver A, Pintado V, Gasch O, Martínez-Martínez L, Pitout J, Akova M, Peña C, Molina J, Hernández A, Venditti M, Prim N, Origüen J, Bou G, Tacconelli E, Tumbarello M, Hamprecht A, Giamarellou H, Almela M, Pérez F, Schwaber MJ, Bermejo J, Lowman W, Hsueh PR, Mora-Rillo M, Natera C, Souli M, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J. A Multinational, Preregistered Cohort Study of β-Lactam/β-Lactamase Inhibitor Combinations for Treatment of Bloodstream Infections Due to Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae. Antimicrob Agents Chemother. 2016 Jul;60(7):4159-69. DOI: 10.1128/AAC.00365-16
[147] Harris PN, Peleg AY, Iredell J, Ingram PR, Miyakis S, Stewardson AJ, Rogers BA, McBryde ES, Roberts JA, Lipman J, Athan E, Paul SK, Baker P, Harris-Brown T, Paterson DL. Meropenem versus piperacillin-tazobactam for definitive treatment of bloodstream infections due to ceftriaxone non-susceptible Escherichia coli and Klebsiella spp (the MERINO trial): study protocol for a randomised controlled trial. Trials. 2015 Jan;16:24. DOI: 10.1186/s13063-014-0541-9
[148] Rosso-Fernández C, Sojo-Dorado J, Barriga A, Lavín-Alconero L, Palacios Z, López-Hernández I, Merino V, Camean M, Pascual A, Rodríguez-Baño J; FOREST Study Group. Fosfomycin versus meropenem in bacteraemic urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli (FOREST): study protocol for an investigator-driven randomised controlled trial. BMJ Open. 2015 Mar;5(3):e007363. DOI: 10.1136/bmjopen-2014-007363
[149] Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011 Mar 1;52(5):e103-20. DOI: 10.1093/cid/ciq257
[150] Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis. 2010 Jan;10(1):43-50. DOI: 10.1016/S1473-3099(09)70325-1
[151] Nakamura T, Komatsu M, Yamasaki K, Fukuda S, Higuchi T, Ono T, Nishio H, Sueyoshi N, Kida K, Satoh K, Toda H, Toyokawa M, Nishi I, Sakamoto M, Akagi M, Mizutani T, Nakai I, Kofuku T, Orita T, Zikimoto T, Natsume S, Wada Y. Susceptibility of various oral antibacterial agents against extended spectrum β-lactamase producing Escherichia coli and Klebsiella pneumoniae. J Infect Chemother. 2014 Jan;20(1):48-51. DOI: 10.1016/j.jiac.2013.08.004
[152] Senol S, Tasbakan M, Pullukcu H, Sipahi OR, Sipahi H, Yamazhan T, Arda B, Ulusoy S. Carbapenem versus fosfomycin tromethanol in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related complicated lower urinary tract infection. J Chemother. 2010 Oct;22(5):355-7. DOI: 10.1179/joc.2010.22.5.355
[153] Rhodes NJ, Gardiner BJ, Neely MN, Grayson ML, Ellis AG, Lawrentschuk N, Frauman AG, Maxwell KM, Zembower TR, Scheetz MH. Optimal timing of oral fosfomycin administration for pre-prostate biopsy prophylaxis. J Antimicrob Chemother. 2015 Jul;70(7):2068-73. DOI: 10.1093/jac/dkv067
[154] Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011 Mar;52(5):e103-20. DOI: 10.1093/cid/ciq257
[155] Grayson ML, Macesic N, Trevillyan J, Ellis AG, Zeglinski PT, Hewitt NH, Gardiner BJ, Frauman AG. Fosfomycin for Treatment of Prostatitis: New Tricks for Old Dogs. Clin Infect Dis. 2015 Oct;61(7):1141-3. DOI: 10.1093/cid/civ436
[156] Wootton M, Walsh TR, Macfarlane L, Howe RA. Activity of mecillinam against Escherichia coli resistant to third-generation cephalosporins. J Antimicrob Chemother. 2010 Jan;65(1):79-81. DOI: 10.1093/jac/dkp404
[157] Lampri N, Galani I, Poulakou G, Katsarolis I, Petrikkos G, Giamarellou H, Souli M. Mecillinam/clavulanate combination: a possible option for the treatment of community-acquired uncomplicated urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli. J Antimicrob Chemother. 2012 Oct;67(10):2424-8. DOI: 10.1093/jac/dks215
[158] Jeppesen N, Frimodt-Møller C. Serum concentrations and penetration into prostate of mecillinam and ampicillin. Curr Med Res Opin. 1984;9(3):213-8. DOI: 10.1185/03007998409109582
[159] Antsupova V, Nørgaard N, Bisbjerg R, Nygaard Jensen J, Boel J, Jarløv JO, Arpi M. Antibiotic prophylaxis for transrectal prostate biopsy-a new strategy. J Antimicrob Chemother. 2014 Dec;69(12):3372-8. DOI: 10.1093/jac/dku293
[160] Naber KG, Niggemann H, Stein G, Stein G. Review of the literature and individual patients' data meta-analysis on efficacy and tolerance of nitroxoline in the treatment of uncomplicated urinary tract infections. BMC Infect Dis. 2014 Nov;14:628. DOI: 10.1186/s12879-014-0628-7
[161] Adams-Haduch JM, Potoski BA, Sidjabat HE, Paterson DL, Doi Y. Activity of temocillin against KPC-producing Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother. 2009 Jun;53(6):2700-1. DOI: 10.1128/AAC.00290-09
[162] Balakrishnan I, Awad-El-Kariem FM, Aali A, Kumari P, Mulla R, Tan B, Brudney D, Ladenheim D, Ghazy A, Khan I, Virgincar N, Iyer S, Carryn S, Van de Velde S. Temocillin use in England: clinical and microbiological efficacies in infections caused by extended-spectrum and/or derepressed AmpC β-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother. 2011 Nov;66(11):2628-31. DOI: 10.1093/jac/dkr317
[163] Balakrishnan I, Smith G. Comment on: The national burden of infections after prostate biopsy in England and Wales: a wake-up call for better prevention. J Antimicrob Chemother. 2013 Oct;68(10):2418-9. DOI: 10.1093/jac/dkt168
[164] Drawz SM, Papp-Wallace KM, Bonomo RA. New β-lactamase inhibitors: a therapeutic renaissance in an MDR world. Antimicrob Agents Chemother. 2014;58(4):1835-46. DOI: 10.1128/AAC.00826-13
[165] Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagacé-Wiens PR, Denisuik A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP 3rd, Karlowsky JA. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Drugs. 2013 Feb;73(2):159-77. DOI: 10.1007/s40265-013-0013-7
[166] Stachyra T, Levasseur P, Péchereau MC, Girard AM, Claudon M, Miossec C, Black MT. In vitro activity of the {beta}-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J Antimicrob Chemother. 2009 Aug;64(2):326-9. DOI: 10.1093/jac/dkp197
[167] Livermore DM, Mushtaq S, Warner M, Miossec C, Woodford N. NXL104 combinations versus Enterobacteriaceae with CTX-M extended-spectrum beta-lactamases and carbapenemases. J Antimicrob Chemother. 2008 Nov;62(5):1053-6. DOI: 10.1093/jac/dkn320
[168] Mushtaq S, Warner M, Livermore DM. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother. 2010 Nov;65(11):2376-81. DOI: 10.1093/jac/dkq306
[169] Sader HS, Castanheira M, Flamm RK, Farrell DJ, Jones RN. Antimicrobial activity of ceftazidime-avibactam against Gram-negative organisms collected from U.S. medical centers in 2012. Antimicrob Agents Chemother. 2014;58(3):1684-92. DOI: 10.1128/AAC.02429-13
[170] Flamm RK, Farrell DJ, Mendes RE, Ross JE, Sader HS, Jones RN. ZAAPS Program results for 2010: an activity and spectrum analysis of linezolid using clinical isolates from 75 medical centres in 24 countries. J Chemother. 2012 Dec;24(6):328-37. DOI: 10.1179/1973947812Y.0000000039
[171] Mawal Y, Critchley IA, Riccobene TA, Talley AK. Ceftazidime-avibactam for the treatment of complicated urinary tract infections and complicated intra-abdominal infections. Expert Rev Clin Pharmacol. 2015;8(6):691-707. DOI: 10.1586/17512433.2015.1090874
[172] Zhanel GG, Chung P, Adam H, Zelenitsky S, Denisuik A, Schweizer F, Lagacé-Wiens PR, Rubinstein E, Gin AS, Walkty A, Hoban DJ, Lynch JP 3rd, Karlowsky JA. Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs. 2014 Jan;74(1):31-51. DOI: 10.1007/s40265-013-0168-2
[173] Sutherland CA, Nicolau DP. Susceptibility Profile of Ceftolozane/Tazobactam and Other Parenteral Antimicrobials Against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa From US Hospitals. Clin Ther. 2015 Jul;37(7):1564-71. DOI: 10.1016/j.clinthera.2015.05.501
[174] Farrell DJ, Sader HS, Flamm RK, Jones RN. Ceftolozane/tazobactam activity tested against Gram-negative bacterial isolates from hospitalised patients with pneumonia in US and European medical centres (2012). Int J Antimicrob Agents. 2014 Jun;43(6):533-9. DOI: 10.1016/j.ijantimicag.2014.01.032
[175] Wagenlehner FM, Umeh O, Steenbergen J, Yuan G, Darouiche RO. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, phase 3 trial (ASPECT-cUTI). Lancet. 2015 May 16;385(9981):1949-56. DOI: 10.1016/S0140-6736(14)62220-0




